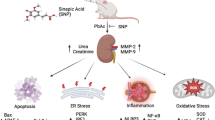
Abstract
Excessive fluoride intake has been reported to cause toxicities to brain, thyroid, kidney, liver and testis tissues. Hesperidin (HSP) is an antioxidant that possesses anti-allergenic, anti-carcinogenic, anti-oxidant and anti-inflammatory activities. Presently, the studies focusing on the toxic effects of sodium fluoride (NaF) on heart tissue at biochemical and molecular level are limited. This study was designed to evaluate the ameliorative effects of HSP on toxicity of NaF on the heart of rats in vivo by observing the alterations in oxidative injury markers (MDA, SOD, CAT, GPX and GSH), pro-inflammatory markers (NF-κB, IL-1β, TNF-α), expressions of apoptotic genes (caspase-3, -6, -9, Bax, Bcl-2, p53, cytochrome c), levels of autophagic markers (Beclin 1, LC3A, LC3B), expression levels of PI3K/Akt/mTOR and cardiac markers. HSP treatment attenuated the NaF-induced heart tissue injury by increasing activities of SOD, CAT and GPx and levels of GSH, and suppressing lipid peroxidation. In addition, HSP reversed the changes in expression of apoptotic (caspase-3, -6, -9, Bax, Bcl-2, p53, cytochrome c), levels of autophagic and inflammatory parameters (Beclin 1, LC3A, LC3B, NF-κB, IL-1β, TNF-α), in the NaF-induced cardiotoxicity. HSP also modulated the gene expression levels of PI3K/Akt/mTOR signaling pathway and levels of cardiac markers (LDH, CK-MB). Overall, these findings reveal that HSP treatment can be used for the treatment of NaF-induced cardiotoxicity.




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Caron, S. (2020). Where does the fluorine come from? A review on the challenges associated with the synthesis of organofluorine compounds. Organic Process Research & Development, 24, 470–480. https://doi.org/10.1021/acs.oprd.0c00030
James, P., Harding, M., Beecher, T., Browne, D., Cronin, M., Guiney, H., O’Mullane, D., & Whelton, H. (2020). Impact of reducing water fluoride on dental caries and fluorosis. Journal of Dental Research, 100, 507–514. https://doi.org/10.1177/0022034520978777
Solanki, Y. S., Agarwal, M., Maheshwari, K., Gupta, S., Shukla, P., & Gupta, A. B. (2021). Removal of fluoride from water by using a coagulant (inorganic polymeric coagulant). Environmental Science and Pollution Research, 28, 3897–3905. https://doi.org/10.1007/s11356-020-09579-2
Caglayan, C., Kandemir, F. M., Darendelioğlu, E., Küçükler, S., & Ayna, A. (2021). Hesperidin protects liver and kidney against sodium fluoride-induced toxicity through anti-apoptotic and anti-autophagic mechanisms. Life Sciences, 281, 119730. https://doi.org/10.1016/j.lfs.2021.119730
Pal, P., & Mukhopadhyay, P. K. (2021). Fluoride induced testicular toxicities in adult Wistar rats. Toxicology Mechanisms and Methods, 31, 383–392. https://doi.org/10.1080/15376516.2021.1891489
Oyagbemi, A. A., Omobowale, T. O., Ola-Davies, O. E., Asenuga, E. R., Ajibade, T. O., Adejumobi, O. A., Afolabi, J. M., Ogunpolu, B. S., Falayi, O. O., Saba, A. B., Adedapo, A. A., & Yakubu, M. A. (2018). Luteolin-mediated Kim-1/NF-kB/Nrf2 signaling pathways protects sodium fluoride-induced hypertension and cardiovascular complications. BioFactors, 44, 518–531. https://doi.org/10.1002/biof.1449
Atmaca, N., Atmaca, H. T., Kanici, A., & Anteplioglu, T. (2014). Protective effect of resveratrol on sodium fluoride-induced oxidative stress, hepatotoxicity and neurotoxicity in rats. Food and Chemical Toxicology, 70, 191–197. https://doi.org/10.1016/j.fct.2014.05.011
Akinrinde, A. S., Tijani, M., Awodele, O. A., & Oyagbemi, A. A. (2021). Fluoride-induced hepatotoxicity is prevented by L-Arginine supplementation via suppression of oxidative stress and stimulation of nitric oxide production in rats. Toxicology and Environmental Health Sciences, 13, 57–64. https://doi.org/10.1007/s13530-020-00070-6
Özbolat, S. N., & Ayna, A. (2021). Chrysin suppresses HT-29 cell death induced by diclofenac through apoptosis and oxidative damage. Nutrition and Cancer, 73, 1419–1428. https://doi.org/10.1080/01635581.2020.1801775
Gulcin, İ. (2020). Antioxidants and antioxidant methods: An updated overview. Archives of toxicology, 94, 651–715. https://doi.org/10.1007/s00204-020-02689-3
Jucá, M. M., Cysne Filho, F. M. S., de Almeida, J. C., Mesquita, D. D. S., Barriga, J. R. D. M., Dias, K. C. F., Barbosa, T. M., Vasconcelos, L. C., Leal, L. K. A. M., Ribeiro, J. E., & Vasconcelos, S. M. M. (2020). Flavonoids: biological activities and therapeutic potential. Natural Product Research, 34, 692–705. https://doi.org/10.1080/14786419.2018.1493588
Kuzu, M., Kandemir, F. M., Yıldırım, S., Çağlayan, C., & Küçükler, S. (2021). Attenuation of sodium arsenite-induced cardiotoxicity and neurotoxicity with the antioxidant, anti-inflammatory, and antiapoptotic effects of hesperidin. Environmental Science and Pollution Research, 28, 10818–10831. https://doi.org/10.1007/s11356-020-11327-5
Caglayan, C., Demir, Y., Kucukler, S., Taslimi, P., Kandemir, F. M., & Gulçin, İ. (2019). The effects of hesperidin on sodium arsenite-induced different organ toxicity in rats on metabolic enzymes as antidiabetic and anticholinergics potentials: a biochemical approach. Journal of Food Biochemistry, 43, e12720. https://doi.org/10.1111/jfbc.12720
Shi, X., Niu, L., Zhao, L., Wang, B., **, Y., & Li, X. (2018). The antiallergic activity of flavonoids extracted from Citri Reticulatae Pericarpium. Journal of Food Processing and Preservation, 42, e13588. https://doi.org/10.1111/jfpp.13588
Pandey, P., & Khan, F. (2021). A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutrition Research, 92, 21–31. https://doi.org/10.1016/j.nutres.2021.05.011
Küçükler, S., Çomaklı, S., Özdemir, S., Çağlayan, C., & Kandemir, F. M. (2021). Hesperidin protects against the chlorpyrifos-induced chronic hepato-renal toxicity in rats associated with oxidative stress, inflammation, apoptosis, autophagy, and up-regulation of PARP-1/VEGF. Environmental Toxicology, 36, 1600–1617. https://doi.org/10.1002/tox.23156
Turk, E., Kandemir, F. M., Yildirim, S., Caglayan, C., Kucukler, S., & Kuzu, M. (2019). Protective effect of hesperidin on sodium arsenite-induced nephrotoxicity and hepatotoxicity in rats. Biological Trace Element Research, 189, 95–108. https://doi.org/10.1007/s12011-018-1443-6
Umarani, V., Muvvala, S., Ramesh, A., Lakshmi, B. V., & Sravanthi, N. (2015). Rutin potentially attenuates fluoride-induced oxidative stress-mediated cardiotoxicity, blood toxicity and dyslipidemia in rats. Toxicology Mechanism and Methods, 25, 143–149. https://doi.org/10.3109/15376516.2014.1003359
Nabavi, S. F., Nabavi, S. M., Mirzaei, M., & Moghaddam, A. H. (2012). Protective effect of quercetin against sodium fluoride induced oxidative stress in rat’s heart. Food & Function, 3, 437–441. https://doi.org/10.1039/C2FO10264A
Sun, Y., Oberley, L. W., & Li, Y. (1988). A simple method for clinical assay of superoxide dismutase. Clinical Chemisty, 34, 497–500. https://doi.org/10.1093/clinchem/34.3.497
Aebi, H. (1984). [13] Catalase in vitro. Methods in Enzymology, 105, 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Lawrence, R. A., & Burk, R. F. (1976). Glutathione peroxidase activity in selenium-deficient rat liver. Biochemical and Biophysical Research Communications, 71, 952–958. https://doi.org/10.1016/0006-291X(76)90747-6
Sedlak, J., & Lindsay, R. H. (1968). Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analitical Biochemistry, 25, 192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Placer, Z. A., Cushman, L. L., & Johnson, B. C. (1966). Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Analitical Biochemistry, 16, 359–364. https://doi.org/10.1016/0003-2697(66)90167-9
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Kucukler, S., Caglayan, C., Darendelioğlu, E., & Kandemir, F. M. (2020). Morin attenuates acrylamide-induced testicular toxicity in rats by regulating the NF-κB, Bax/Bcl-2 and PI3K/Akt/mTOR signaling pathways. Life Sciences, 261, 118301. https://doi.org/10.1016/j.lfs.2020.118301
Tartik, M., Darendelioglu, E., Aykutoglu, G., & Baydas, G. (2016). Turkish propolis supresses MCF-7 cell death induced by homocysteine. Biomedicine and Pharmacotherapy, 82, 704–712. https://doi.org/10.1016/j.biopha.2016.06.013
Song, C., Shi, D., Chang, K., Li, X., Dong, Q., Ma, X., Wang, X., Guo, Z., Liu, Y., & Wang, J. (2021). Sodium fluoride activates the extrinsic apoptosis via regulating NOX4/ROS-mediated p53/DR5 signaling pathway in lung cells both in vitro and in vivo. Free Radical Biology and Medicine, 169, 137–148. https://doi.org/10.1016/j.freeradbiomed.2021.04.007
Brillo, V., Chieregato, L., Leanza, L., Muccioli, S., & Costa, R. (2021). Mitochondrial dynamics, ROS, and cell signaling: a blended overview. Life. https://doi.org/10.3390/life11040332
Herb, M., & Schramm, M. (2021). Functions of ROS in macrophages and antimicrobial immunity. Antioxidants. https://doi.org/10.3390/antiox10020313
Schieber, M., & Chandel, N. S. (2014). ROS function in redox signaling and oxidative stress. Current Biology, 24, R453–R462. https://doi.org/10.1016/j.cub.2014.03.034
Kucukler, S., Darendelioğlu, E., Caglayan, C., Ayna, A., Yıldırım, S., & Kandemir, F. M. (2020). Zingerone attenuates vancomycin-induced hepatotoxicity in rats through regulation of oxidative stress, inflammation and apoptosis. Life Sciences, 259, 118382. https://doi.org/10.1016/j.lfs.2020.118382
Caglayan, C., Kandemir, F. M., Yildirim, S., Kucukler, S., & Eser, G. (2019). Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. Journal of Trace Element Medicine Biology, 54, 69–78. https://doi.org/10.1016/j.jtemb.2019.04.007
Nkpaa, K. W., & Onyeso, G. I. (2018). Rutin attenuates neurobehavioral deficits, oxidative stress, neuro-inflammation and apoptosis in fluoride treated rats. Neuroscience Letters, 682, 92–99. https://doi.org/10.1016/j.neulet.2018.06.023
Bouasla, A., Barour, C., Bouasla, I., & Messarah, M. (2021). Beneficial effects of Punica granatum l. juice and gallic acid against kidney oxidative damage caused by sodium fluoride. Pharmaceutical Chemistry Journal, 55, 920–928. https://doi.org/10.1007/s11094-021-02516-8
Yamaguti, P. M., Simões, A., Ganzerla, E., Souza, D. N., Nogueira, F. N., & Nicolau, J. (2013). Effects of single exposure of sodium fluoride on lipid peroxidation and antioxidant enzymes in salivary glands of rats. Oxidative Medicine and Cellular Longevity, 2013, 674593. https://doi.org/10.1155/2013/674593
Semis, H. S., Kandemir, F. M., Kaynar, O., Dogan, T., & Arikan, S. M. (2021). The protective effects of hesperidin against paclitaxel-induced peripheral neuropathy in rats. Life Sciences, 287, 120104. https://doi.org/10.1016/j.lfs.2021.120104
Volpe, C. M. O., Villar-Delfino, P. H., dos Anjos, P. M. F., & Nogueira-Machado, J. A. (2018). Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death & Disease, 9, 119. https://doi.org/10.1038/s41419-017-0135-z
Yu, H., Lin, L., Zhang, Z., Zhang, H., & Hu, H. (2020). Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduction and Targeted Therapy, 5, 209. https://doi.org/10.1038/s41392-020-00312-6
Chen, L., Kuang, P., Liu, H., Wei, Q., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L. (2019). Sodium fluoride (NaF) induces inflammatory responses via activating MAPKs/NF-κB signaling pathway and reducing anti-inflammatory cytokine expression in the mouse liver. Biological Trace Element Research, 189, 157–171. https://doi.org/10.1007/s12011-018-1458-z
Holze, C., Michaudel, C., Mackowiak, C., Haas, D. A., Benda, C., Hubel, P., Pennemann, F. L., Schnepf, D., Wettmarshausen, J., Braun, M., Leung, D. W., Amarasinghe, G. K., Perocchi, F., Staeheli, P., Ryffel, B., & Pichlmair, A. (2018). Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nature Immunology, 19, 130–140. https://doi.org/10.1038/s41590-017-0013-y
Gao, J., Tian, X., Yan, X., Wang, Y., Wei, J., Wang, X., Yan, X., & Song, G. (2021). Selenium exerts protective effects against fluoride-induced apoptosis and oxidative stress and altered the expression of Bcl-2/Caspase family. Biological Trace Element Research, 199, 682–692. https://doi.org/10.1007/s12011-020-02185-w
Wei, Q., Luo, Q., Liu, H., Chen, L., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L. (2018). The mitochondrial pathway is involved in sodium fluoride (NaF)-induced renal apoptosis in mice. Toxicology Research, 7, 792–808. https://doi.org/10.1039/c8tx00130h
Rodius, S., de Klein, N., Jeanty, C., Sánchez-Iranzo, H., Crespo, I., Ibberson, M., Xenarios, I., Dittmar, G., Mercader, N., Niclou, S. P., & Azuaje, F. (2020). Fisetin protects against cardiac cell death through reduction of ROS production and caspases activity. Scientific Reports, 10, 2896. https://doi.org/10.1038/s41598-020-59894-4
Benzer, F., Kandemir, F. M., Ozkaraca, M., Kucukler, S., & Caglayan, C. (2018). Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. Journal of Biochemical and Molecular Toxicology, 32, e22030. https://doi.org/10.1002/jbt.22030
Yang, H., **ng, R., Liu, S., Yu, H., & Li, P. (2019). Analysis of the protective effects of γ-aminobutyric acid during fluoride-induced hypothyroidism in male Kunming mice. Pharmaceutical Biology, 57, 28–36. https://doi.org/10.1080/13880209.2018.1563621
Hoxhaj, G., & Manning, B. D. (2020). The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nature Reviews Cancer, 20, 74–88. https://doi.org/10.1038/s41568-019-0216-7
Korkmaz, R., Yüksek, V., & Dede, S. (2021). The effects of sodium fluoride (NaF) treatment on the PI3K/Akt signal pathway in NRK-52E cells. Biological Trace Element Research. https://doi.org/10.1007/s12011-021-02927-4
Ma, L., Zhang, R., Li, D., Qiao, T., & Guo, X. (2021). Fluoride regulates chondrocyte proliferation and autophagy via PI3K/AKT/mTOR signaling pathway. Chemico-Biological Interactions, 349, 109659. https://doi.org/10.1016/j.cbi.2021.109659
Li, X., Hu, X., Wang, J., Xu, W., Yi, C., Ma, R., & Jiang, H. (2018). Inhibition of autophagy via activation of PI3K/Akt/mTOR pathway contributes to the protection of hesperidin against myocardial ischemia/reperfusion injury. International Journal of Molecular Medicine, 42, 1917–1924. https://doi.org/10.3892/ijmm.2018.3794
Kandemir, F. M., Yıldırım, S., Kucukler, S., Caglayan, C., Darendelioğlu, E., & Dortbudak, M. B. (2020). Protective effects of morin against acrylamide-induced hepatotoxicity and nephrotoxicity: A multi-biomarker approach. Food and Chemical Toxicology, 138, 111190. https://doi.org/10.1016/j.fct.2020.111190
He, C., & Levine, B. (2010). The beclin 1 interactome. Current Opinion in Cell Biology, 22, 140–149. https://doi.org/10.1016/j.ceb.2010.01.001
Kang, R., Zeh, H. J., Lotze, M. T., & Tang, D. (2011). The Beclin 1 network regulates autophagy and apoptosis. Cell Death & Differentiation, 18, 571–580. https://doi.org/10.1038/cdd.2010.191
Koukourakis, M. I., Kalamida, D., Giatromanolaki, A., Zois, C. E., Sivridis, E., Pouliliou, S., Mitrakas, A., Gatter, K. C., & Harris, A. L. (2015). Autophagosome proteins LC3A, LC3B and LC3C have distinct subcellular distribution kinetics and expression in cancer cell lines. PLoS ONE, 10, e0137675. https://doi.org/10.1371/journal.pone.0137675
Gur, C., Kandemir, O., & Kandemir, F. M. (2022). Investigation of the effects of hesperidin administration on abamectin-induced testicular toxicity in rats through oxidative stress, endoplasmic reticulum stress, inflammation, apoptosis, autophagy, and JAK2/STAT3 pathways. Environmental Toxicology, 37, 401–412. https://doi.org/10.1002/tox.23406
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BV, CC and FMK designed the research. ED, ÖK and AG conducted experiments. ED, BV, FMK and AA analyzed data. BV, CC and AA wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest. The authors report no declarations of interest.
Ethical approval
The experimental protocol was approved by the Animal Experiments Local Ethics Committee of the Bingol University (Protocol No. 2020-E.8227).
Consent to participate
Not applicable.
Additional information
Handling Editor: Lorraine Chalifour.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Varışlı, B., Darendelioğlu, E., Caglayan, C. et al. Hesperidin Attenuates Oxidative Stress, Inflammation, Apoptosis, and Cardiac Dysfunction in Sodium Fluoride‐Induced Cardiotoxicity in Rats. Cardiovasc Toxicol 22, 727–735 (2022). https://doi.org/10.1007/s12012-022-09751-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-022-09751-9