Abstract
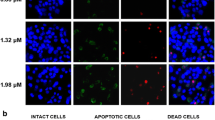
Based on the various pharmacological activities of tamarixetin, the present study investigated the cytotoxicity property of tamarixetin in human liver cancer cells including PLC/PRF/5 and HepG2 cells, and their xenografted tumor nude mice. In cells, tamarixetin incubation resulted in the suppression on cell viability; enhanced cell apoptosis rate, LDH release, caspase-3 activation, and reactive oxygen species accumulation; and decreased mitochondrial membrane potential in a dose-dependent manner. Tamarixetin inhibited the growth of PLC/PRF/5- and HepG2-xenografted tumors in BALB/c nude mice after 14-day administration without influencing their bodyweights and organ functions including liver and spleen. Tamarixetin enhanced the expression levels of pro-apoptotic proteins including Bax and cleaved caspase-3 and inhibited the expression levels of anti-apoptotic proteins including Bcl-2 and Bcl-xL in liver cancer cells and their xenografted tumor tissues. Furthermore, tamarixetin significantly suppressed the phosphorylation of ERKs and AKT in both PLC/PRF/5 and HepG2 cells, and tumor tissues. All present data suggest that tamarixetin displays pro-apoptotic properties in liver cancer cells related to the mitochondria apoptotic pathway via regulating the ERKs and AKT signaling.







Similar content being viewed by others
Availability of Data and Materials
All data generated and analyzed during the present study are included in this published article.
Abbreviations
- AKT:
-
Protein kinase B
- Bax:
-
Bcl-2-associated X protein
- Bcl-2:
-
B cell lymphoma 2
- Bcl-xL:
-
B cell lymphoma-extra large
- BSA:
-
Bovine serum albumin
- DCFH-DA:
-
2,7-dichlorofluorescein diacetate
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- ERKs:
-
Extracellular signal-regulated kinases
- FBS:
-
Fetal bovine serum
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- JC-1:
-
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- LDH:
-
Lactate dehydrogenase
- MAPKs:
-
Mitogen-activated protein kinases
- MMP:
-
Mitochondrial membrane potential
- MPTP:
-
Mitochondrial permeability transition pores
- MTT:
-
3-(4,5)-dimethylthiahiazo(−z-y1)-3,5-di-pheny-tetrazoliumromide
- RIPA:
-
Radio Immunoprecipitation Assay
- ROS:
-
Reactive oxygen species
- TBS-T:
-
Tris-buffered saline-Tween
References
Llovet, J. M., Burroughs, A., & Bruix, J. (2003). Hepatocellular carcinoma. Lancet, 362(9399), 1907–1917.
Jia, Z., Yang, H. H., Liu, Y. J., & Wang, X. Z. (2018). Synthetic dibenzoxanthene derivatives induce apoptosis through mitochondrial pathway in human hepatocellular cancer cells. Applied Biochemistry and Biotechnology, 186(1), 145–160.
Li, C. J., Huang, S. Y., Wu, M. Y., Chen, Y. C., Tsang, S. F., Chyuan, J. H., & Hsu, H. Y. (2012). Induction of apoptosis by ethanolic extract of Corchorus olitorius leaf in human hepatocellular carcinoma (HepG2) cells via a mitochondria-dependent pathway. Molecules, 17(8), 9348–9360.
Liu, Y. M., Liu, Y. K., Lan, K. L., Lee, Y. W., Tsai, T. H., & Chen, Y. J. (2013). Medicinal fungus Antrodia cinnamomea inhibits growth and cancer stem cell characteristics of hepatocellular carcinoma. Evidence-based Complementary and Alternative Medicine, 2013, 569737.
Wang, D., Wong, H. K., Feng, Y. B., & Zhang, Z. J. (2014). 18beta-glycyrrhetinic acid induces apoptosis in pituitary adenoma cells via ROS/MAPKs-mediated pathway. Journal of Neuro-Oncology, 116(2), 221–230.
Wang, D., Lu, J., Liu, Y., Meng, Q., **e, J., Wang, Z., & Teng, L. (2014). Liquiritigenin induces tumor cell death through mitogen-activated protein kinase- (MPAKs-) mediated pathway in hepatocellular carcinoma cells. BioMed Research International, 2014, 965316.
Dias, N., & Bailly, C. (2005). Drugs targeting mitochondrial functions to control tumor cell growth. Biochemical Pharmacology, 70(1), 1–12.
Jonas, E. (2006). BCL-xL regulates synaptic plasticity. Molecular Interventions, 6(4), 208–222.
Hu, K. H., Li, W. X., Sun, M. Y., Zhang, S. B., Fan, C. X., Wu, Q., Zhu, W., & Xu, X. (2015). Cadmium induced apoptosis in MG63 cells by increasing ROS, activation of p38 MAPK and inhibition of ERK 1/2 pathways. Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 36, 642–654.
Wang, D., Guo, T. Q., Wang, Z. Y., Lu, J. H., Liu, D. P., Meng, Q. F., **e, J., Zhang, X. L., Liu, Y., & Teng, L. S. (2014). ERKs and mitochondria-related pathways are essential for glycyrrhizic acid-mediated neuroprotection against glutamate-induced toxicity in differentiated PC12 cells. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas, 47, 773–779.
Hayamizu, K., Morimoto, S., Nonaka, M., Hoka, S., & Sasaguri, T. (2018). Cardiotonic actions of quercetin and its metabolite tamarixetin through a digitalis-like enhancement of Ca(2+) transients. Archives of Biochemistry and Biophysics, 637, 40–47.
Yadav, D. K., Bharitkar, Y. P., Hazra, A., Pal, U., Verma, S., Jana, S., Singh, U. P., Maiti, N. C., Mondal, N. B., & Swarnakar, S. (2017). Tamarixetin 3-O-beta-d-Glucopyranoside from Azadirachta indica leaves: Gastroprotective role through inhibition of matrix metalloproteinase-9 activity in mice. Journal of Natural Products, 80(5), 1347–1353.
Park, H. J., Lee, S. J., Cho, J., Gharbi, A., Han, H. D., Kang, T. H., Kim, Y., Lee, Y., Park, W. S., Jung, I. D., & Park, Y. M. (2018). Tamarixetin exhibits anti-inflammatory activity and prevents bacterial sepsis by increasing IL-10 production. Journal of Natural Products, 81(6), 1435–1443.
Nicolini, F., Burmistrova, O., Marrero, M. T., Torres, F., Hernandez, C., Quintana, J., & Estevez, F. (2014). Induction of G2/M phase arrest and apoptosis by the flavonoid tamarixetin on human leukemia cells. Molecular Carcinogenesis, 53(12), 939–950.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65(1-2), 55–63.
Song, J., Wang, Y., Teng, M., Zhang, S., Yin, M., Lu, J., Liu, Y., Lee, R. J., Wang, D., & Teng, L. (2016). Cordyceps militaris induces tumor cell death via the caspasedependent mitochondrial pathway in HepG2 and MCF7 cells. Molecular Medicine Reports, 13(6), 5132–5140.
Zhang, X., Chen, Y., Cai, G., Li, X., & Wang, D. (2017). Carnosic acid induces apoptosis of hepatocellular carcinoma cells via ROS-mediated mitochondrial pathway. Chemico-Biological Interactions, 277, 91–100.
Pearson, G., Robinson, F., Beers Gibson, T., Xu, B. E., Karandikar, M., Berman, K., & Cobb, M. H. (2001). Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews, 22(2), 153–183.
Jose, G. M., Raghavankutty, M., & Kurup, G. M. (2018). Sulfated polysaccharides from Padina tetrastromatica induce apoptosis in HeLa cells through ROS triggered mitochondrial pathway. Process Biochemistry, 68, 197–204.
Kang, J. H., Zhang, W. Q., Song, W., Shen, D. Y., Li, S. S., Tian, L., Shi, Y., Liang, G., **ong, Y. X., & Chen, Q. X. (2012). Apoptosis mechanism of human cholangiocarcinoma cells induced by bile extract from crocodile. Applied Biochemistry and Biotechnology, 166(4), 942–951.
Hippe, D., Gais, A., Gross, U., & Luder, C. G. (2009). Modulation of caspase activation by toxoplasma gondii. Methods in Molecular Biology, 470, 275–288.
Porter, A. G., & Janicke, R. U. (1999). Emerging roles of caspase-3 in apoptosis. Cell Death and Differentiation, 6(2), 99–104.
Ding, J., Mooers, B. H., Zhang, Z., Kale, J., Falcone, D., McNichol, J., Huang, B., Zhang, X. C., **ng, C., Andrews, D. W., & Lin, J. (2014). After embedding in membranes antiapoptotic Bcl-XL protein binds both Bcl-2 homology region 3 and helix 1 of proapoptotic Bax protein to inhibit apoptotic mitochondrial permeabilization. The Journal of Biological Chemistry, 289(17), 11873–11896.
Brown, D. I., & Griendling, K. K. (2015). Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circulation Research, 116(3), 531–549.
Circu, M. L., & Aw, T. Y. (2010). Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Biology & Medicine, 48(6), 749–762.
Wang, H., & Xu, W. (2017). Mito-methyl coumarin, a novel mitochondria-targeted drug with great antitumor potential was synthesized. Biochemical and Biophysical Research Communications, 489(1), 1–7.
Zhao, Y., Guo, C., Wang, L., Wang, S., Li, X., Jiang, B., Wu, N., Guo, S., Zhang, R., Liu, K., & Shi, D. (2017). A novel fluorinated thiosemicarbazone derivative- 2-(3,4-difluorobenzylidene) hydrazinecarbothioamide induces apoptosis in human A549 lung cancer cells via ROS-mediated mitochondria-dependent pathway. Biochemical and Biophysical Research Communications, 491(1), 65–71.
Sweatt, J. D. (2001). The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. Journal of Neurochemistry, 76(1), 1–10.
Krajarng, A., Nakamura, Y., Suksamrarn, S., & Watanapokasin, R. (2011). Alpha-Mangostin induces apoptosis in human chondrosarcoma cells through downregulation of ERK/JNK and Akt signaling pathway. Journal of Agricultural and Food Chemistry, 59(10), 5746–5754.
Tada, K., Kawahara, K., Matsushita, S., Hashiguchi, T., Maruyama, I., & Kanekura, T. (2012). MK615, a Prunus mume Steb. Et Zucc (‘Ume’) extract, attenuates the growth of A375 melanoma cells by inhibiting the ERK1/2-Id-1 pathway. Phytotherapy Research, 26(6), 833–838.
Zhao, Y., Shen, S., Guo, J., Chen, H., Greenblatt, D. Y., Kleeff, J., Liao, Q., Chen, G., Friess, H., & Leung, P. S. (2006). Mitogen-activated protein kinases and chemoresistance in pancreatic cancer cells. The Journal of Surgical Research, 136(2), 325–335.
Balmanno, K., & Cook, S. J. (2009). Tumour cell survival signalling by the ERK1/2 pathway. Cell Death and Differentiation, 16(3), 368–377.
**, Y., Yan, E. Z., Fan, Y., Guo, X. L., Zhao, Y. J., Zong, Z. H., & Liu, Z. (2007). Neuroprotection by sodium ferulate against glutamate-induced apoptosis is mediated by ERK and PI3 kinase pathways. Acta Pharmacologica Sinica, 28(12), 1881–1890.
Shao, M., He, Z., Yin, Z., Ma, P., **ao, Q., Song, Y., Huang, Z., Ma, Y., Qiu, Y., Zhao, A., Zhou, T., & Wang, Q. (2018). **huang pill induces apoptosis of human glioblastoma U-87 MG cells via targeting ROS-mediated Akt/mTOR/FOXO1 pathway. Evidence-based complementary and alternative medicine : eCAM, 2018, 6049498.
Funding
This work was supported by the National Nature Science Foundation of China (NO. 81501420) and the Special Projects of Cooperation between Jilin University and Jilin Province in P. R. China (SXGJSF2017-1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The experimental animal study was approved by Jilin University (2017–0110).
Patient Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, J., Cai, X., Teng, S. et al. The Pro-Apoptotic Activity of Tamarixetin on Liver Cancer Cells Via Regulation Mitochondrial Apoptotic Pathway. Appl Biochem Biotechnol 189, 647–660 (2019). https://doi.org/10.1007/s12010-019-03033-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03033-x