Abstract
Agricultural products are perishable and easily contaminated by spoilage microorganisms or foodborne pathogens, causing decay of food and illness in consumers. Chlorine dioxide (ClO2) has many advantages as a fungicide in comparison to chlorine, NaClO, and ozone, and it can be used for sterilization and preservation due to its strong oxidizing properties. ClO2 gas is more widely used in food safety and food preservation than its aqueous solutions. However, the ClO2 gas produced on a large scale from a chemical reactor cannot be applied to food in small packages. Several recent reports have discussed the use of ClO2 self-releasing packaging. This article highlights the systematic evaluation of the performance of ClO2 alone and in combination with other treatments, such as 1-MCP, chitosan, and UV irradiation, in maintaining food safety and freshness of agricultural products. This article also focuses on the biological functions and mechanisms of ClO2-mediated preservation of plant or animal food, including damage to the cell wall and the membrane of pathogens, inhibition of ethylene biosynthesis, restoration of redox balance, and change of the compound structure of toxins, such as pesticides, insecticides, and mycotoxins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food safety is an area of major focus worldwide. Agricultural products are the most important source of our daily food. These products are highly perishable due to environmental changes and are easily contaminated by microorganisms through pre-harvest and wound infection as well as by human pathogens during the different steps of food production, such as processing, transportation, and packaging. Pathogens can cause foodborne illness outbreak and induce an unfavorable impact on the development of the entire food industry. Consequently, various antimicrobial agents are needed to ensure food safety and maintain food quality. To reduce microbial load and to better maintain freshness of agricultural products, sodium hypochlorite (NaClO) is commonly used as a disinfectant due to its low cost and ease of use; however, its toxicity and loss of effectivity with time limit its use (Duan et al. 2016; Zhang and Fu 2018). Chorine (Cl2) is also widely applied in the food industry and has broad spectrum antibacterial properties (Ramos et al. 2013). However, it is corrosive and produces toxic by-products (carcinogenic and mutagenic chlorinated compounds) upon reacting with other organic compounds (Bull et al. 2011; Legay et al. 2010). Besides, it has limited efficiency in decreasing the microbial population (Yaron and Römling 2014). Ozone is an efficient bactericide and preservative at only small concentrations ranging from 1 to 5 mg L−1 when dissolved in water. However, it can cause discoloration and deterioration of product quality and produce irritation, which can harm the human body (Artés et al. 2009; Sun et al. 2018). Chlorine dioxide (ClO2) is an efficient disinfectant that has a strong oxidation capacity at low concentrations (0.1 mg L−1) (Artés et al. 2009) and in a wide pH range (pH 3–8) (Aieta and Berg 1986; Huang et al. 1997; Ölmez and Kretzschmar 2009).
At room temperature, ClO2 exists as a yellowish to reddish gas that readily dissolves in water at large concentrations. The gas has an irritating nature and its color changes as the concentration increases (WHO 2000). However, it is unstable and explosive at high concentrations (more than 10% v/v) and cannot be stored and transported over long distances (Deshwal and Lee 2004; Wang and Hu 2008). ClO2 is commonly utilized as a bleaching agent in water treatment plants, paper industry, etc. It is also employed as a disinfectant in laboratories, hospitals, public places, and other areas (Gómez-López et al. 2009). ClO2 is identified as a highly efficient antibacterial agent, bleaching agent, and antioxidant. In USA, Food and Drug Administration (FDA) approved it as a disinfectant for agricultural products due to its safe and efficient properties (FDA 2008).
Many authors reported the applications of gaseous and dissolved ClO2 in food safety and preservation. Sun et al. (2018) chiefly evaluated the action mechanisms of ClO2 against microorganisms and the effects of gaseous ClO2 treatment on quality of fruit and vegetables. Praeger et al. (2016) mainly reported that the impacts of aqueous ClO2 alone or combined with other treatments on microorganisms and nutrients for horticultural produce. However, other biological functions and mechanisms of ClO2 in plant or animal food safety and preservation as well as the range of ClO2 applications have not been widely reported.
In this review article, we summarized the current knowledge regarding the production and the applications of both gaseous and dissolved ClO2 in ensuring safety and quality of food, including fruits, vegetables, dairy products, edible flowers, food crops, aquatic products, condiments, livestock, and poultry products. This article also discusses the use of ClO2 in the inhibition of the ripening of green pepper and sprouting of potato tubers, thereby improving their shelf lives.
Production Methods for and Action Mechanisms of Chlorine Dioxide
Production Methods
ClO2 gas is widely used in food decontamination due to its high penetration ability compared to its aqueous solutions (Gómez-López et al. 2009; Han et al. 2001). Practically, ClO2 is, e.g., produced with a chemical reactor using a chemical negative pressure aeration process (Wang and Guo 2001) employing hydrochloric acid reacted with either sodium chlorite or sodium chlorate (Tan 2018). In addition, ClO2 is also produced with an electrolytic reactor by the decomposition of aqueous solutions of NaCl via a special diaphragm electrolyzer (Li et al. 2014). The practical use of the latter is, however, limited because of the relative low purity of generated gas (Deshwal and Lee 2004; Li et al. 2014). And, in fact, there are many systems commercially available. As shown in Fig. 1, a typical chemical generator consists mainly of two parts: the reactor, which contains a mixture of sodium chlorite or sodium chlorate and reducing substances, such as sulfur dioxide, methanol, oxalic acid, and hydrogen peroxide and the absorber, which collects the gases by absorbing solution (ca. 2% carbonate buffered potassium iodide) (Deshwal and Lee 2004).
Several other methods of ClO2 production have been practically used. The production of gaseous ClO2 involves a direct reaction between sodium chlorite and solid acid, but the speed and amount of ClO2 ejected from the reactor cannot be controlled (Li et al. 2012a). The production efficacy of ClO2 gas depends on several factors, such as pH, temperature, concentration, humidity (Shirasaki et al. 2016), and production methods.
Wu et al. (2010) reported a ClO2-based food preservative, i.e., ClO2 generated by reducing chlorate using sulfite (Na2S2O5) to release SO2 gas. Deshwal and Lee (2005) suggested a multilayered bio-based packaging material that may directly generated ClO2 from NaClO2 and an acid within food packages. Saade et al. (2018) proposed the combination of functional layers of pectin (contains citric acid) and gelatin (sodium chlorite) as well as optional barrier gelation layers (without sodium chlorite) inserted between the two. The theoretical yield of ClO2 gas calculated via this activation was up to 81%, which is a considerably optimization in concentration and release-rate of ClO2 compared with the method mentioned by Saade et al. (2017). Similarly, Zhou et al. (2018) proposed another approach in which the respirational carbon dioxide and water vapor released from fresh fruit form carbonic acid, which then could react with sodium chlorite (NaClO2) to generate ClO2 gas under acidic conditions.
Antimicrobial Mechanisms
The sanitation mechanisms of ClO2 may be based on its strong oxidizing power, which is over 2.5 times that of chlorine in HOCl (Wu and Rioux 2010). The capacity of oxidation depends on the structural characteristics. ClO2 molecule has 19 valence electrons and an unpaired valence electron, which exists as a monomeric free radical. Free radicals themselves have strong oxidative properties, thus causing the strong oxidizing ability of ClO2 (Shan and Lu 2013).
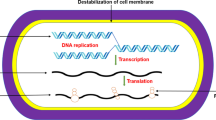
The cell membrane is recognized as a significant target of ClO2 against microorganisms. ClO2 gas can permeate and oxidize proteins localized in the cell surface, resulting in the metabolism disorder of the cells (Vandekinderen et al. 2009; Sun et al. 2018). Moreover, ClO2 not only penetrates the microbial cell membrane and inhibits respiration and deteriorates the trans-membrane ion gradients (Berg et al. 1986), it also modifies the conformation of proteins and lipids of the outer membrane (Praeger et al. 2016).
ClO2 can oxidize amino acids, especially tyrosine, thus affecting the synthesis of microbial proteins and microbial metabolism (Fu and Du 2004). ClO2 was found to oxidize the mercapto groups of enzymes to develop disulfide analogs, damaging the normal physiological function of those enzymes (Huang et al. 1997). In addition, ClO2 may also damage protein by oxidation, causing genotoxicity (Buschini et al. 2004), degradation of viral RNA (Simonet and Gantzer 2006), disruption of important compounds in biochemical reactions (Sharma and Sohn 2012), and thereby mediating microbial death (Sun et al. 2018).
Guntiyaa et al. (2016) reported that electrolyte leakage, the activation of the pathogen cell wall damaging enzymes chitinase (CHI) and glucanase (GLU), and the inhibition of mycelial growth and spore germination were vital events in controlling Cladosporium spp., Fusarium spp., and Lasiodiplodia spp. on harvested ‘Daw’ logan fruit with ClO2 gaseous treatment.
In addition, Zhang and Fu (2018) noted that ClO2 significantly reduced the diameter of lesions resulting from Penicillium expansum, a destructive fungus, causing severe fruit rot. The effect of ClO2 treatment was more obvious as the concentration (0–200 mg L−1) of ClO2 was increased. The mycelial growth, germ tube length, spore germination rate, and spore yields of P. expansum were reduced. Besides, the mycelial morphology, the integrity of plasma membranes, and the mitochondrial membrane potential were changed after ClO2 treatment, as observed through scanning electron microscopy (SEM), PI, and rhodamine 123 staining. Referring to the mechanism of inactivation of fungi, Wen et al. (2017) noted that the fungal spores in groundwater were effectively inactivated due to the leakage of intracellular substances, increase in extracellular substances (adenosine triphosphate/DNA/proteins), and change in shape from smooth to rough as observed through SEM.
Preservation Mechanisms
Ethylene is essential to regulate the physiological and biochemical changes that occur during maturation and senescence of fruits (Bleecker et al. 1988). Therefore, inhibition of ethylene biosynthesis is beneficial for the preservation of fruits and vegetables after harvest. Ethylene is enzymatically synthesized from the amino acid methionine via the intermediated steps S-adenosyl-L-methionine (SAM) and 1-aminocyclopropane-1-carboxylic acid (ACC) (Adams and Yang 1979). ClO2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (Fu and Du 2004). Guo et al. (2014b) suggested that ClO2 inhibited ethylene biosynthesis by down regulating the relevant genes (LeACS2, LeACS4, and LeACO1) in harvested tomatoes.
A stable adenylate metabolism would guarantee a prolonged metabolic activity in fruits. Chumyam et al. (2016) reported that treating longan fruit with gaseous ClO2 activated respiration-related enzymes like succinate dehydrogenase (SDH) and cytochrome c oxidase (CCO) during storage besides restoring the cellular redox balance via increasing the ratios of pyridine nucleotide (NAD/NADH) and ubiquinone (Q/QH2) to maintain quality and extend the shelf life of the fruit.
Toxins Degradation Mechanisms
ClO2 degraded mycotoxins by decreasing the production of toxins or damaging their structure. Patulin, a secondary product, is produced by P. expansum and has violent toxicity in humans and animals (Barad et al. 2014). Zhang and Fu (2018) noted that patulin production in both apple juice and potato dextrose broth (PDB) medium was observably decreased as the concentration (100–400 mg L−1) of ClO2 increased. Nevertheless, the degradation rate of pure patulin in an apple juice reduced slightly after ClO2 treatment at different concentrations (Zhang et al. 2018a).
The mechanism of ClO2 action in degrading trichothecene mycotoxins mainly includes (1) ClO2 oxidized the epoxide group in the trichothecene structure, opening the ring thus destroying the whole molecule structure (Swanson et al. 1988). (2) The major chemical groups in trichothecene, such as oxygen and hydroxyl groups, are altered by the oxidation. (3) ClO2 solution had a better effect on the removal of mycotoxins than its gaseous form due to the increase in production of free radicals (Wilson et al. 2005).
ClO2 degrades pesticides (tebuconazole) by damaging the C–C bond linked to the tertiary OH group and producing the tertiary butyl cation and p-chlorobenzyl, thereby distorting the structure of the compound completely. The removal of E and Z isomers of dimethomorph was reported by Wei et al. (2018). Similarly, Tian et al. (2014) noted that phenylurea herbicides (chlortoluron, isoproturon, and diuron) were degraded by ClO2-mediated oxidation followed by electron transfer, chemical bond cleavage, and atomic substitution.
Chloral hydrate, an important drinking water disinfection by-product, was removed up to 14.3% at 0.4 mg L−1 ClO2 owing to its ability to oxidize hydrophilic substances, besides also oxidizing the C=C and C=O bonds of unsaturated aromatic substances and microbial metabolites (Huang et al. 2018).
Application of Chlorine Dioxide in Food Safety
No Lasting Chemical Residues Production
In the process of application, ClO2 forms much chloride, but little chlorite and/or chlorate. The hypothesis that application of aqueous and gaseous ClO2 does not cause lasting chemical residues (ClO2, ClO2−, and ClO3−) in fruits and vegetables is reasonable (Praeger et al. 2016). Chen et al. (2011) reported that no residues were detected in plum fruits following a 10-min treatment with a combination of aqueous ClO2 (40 mg L−1) and ultrasonic treatment (100 W). In addition, Smith et al. (2015) noted that in tomatoes and cantaloupes treated by gas 36ClO2, chlorate residues are found at concentrations of less than 60 and 30 ng g−1, respectively, and there was no significant difference between the treated and control products.
Human and Plant Pathogens Elimination
It has been reported that ClO2 can control bacterial pathogens such as Escherichia coli O157:H7, Alternaria alternata, Salmonella enterica, Listeria monocytogenes, and Bacillus subtilis in fresh food (Smith et al. 2015; Sun et al. 2017c). Kim and Song (2017) mentioned that the load of L. monocytogenes and E. coli O157:H7 inoculated onto plum fruits was lowered by 6.26 and 5.48 log CFU g−1, respectively, after 20-min treatment with 0.08 mg L−1 ClO2 gas, 0.5% fumaric acid, and UV-C (10 kJ m−2). Moreover, Cho et al. (2017) noted that E. coli, Salmonella typhimurium, and L. monocytogenes were reduced on the surface of carrots washed for 30 min with 100 to 400 mg L−1 aerosolized ClO2, and decontamination effects increased with the ClO2 concentration. In addition, a more than 5 log CFU per potato reduction of natural microorganisms was achieved after a 5-h treatment with 40 mg L−1 of gaseous ClO2 (Wu and Rioux 2010). Pathogens (E. coli, S. typhimurium, and L. monocytogenes) inoculated on the leaves of spinach were reduced to below detection limit (1 log CFU g−1) within 15 min of treatment with ClO2 gas at 0.13 mg L−1 under 90% relative humidity (Park and Kang 2015). Park et al. (2018a) indicated that the inhibiting effect of ClO2 treatment on three pathogens (E. coli, S. typhimurium, and L. monocytogenes) was connected to RH (50–90%), ClO2 concentration (0.01–0.08 mg L−1), and treatment time in tomatoes.
E. coli and S. enteritidis on alfalfa and mung bean sprouts were inactivated by a combination treatment of aqueous ClO2 and ultrasound, which was more effective than when each one was used alone (Millan-Sangoa et al. 2017). Additionally, Kim et al. (2009) reported that 50 mg L−1 aqueous ClO2 combined with 0.5% fumaric acid significantly had a better effect against two common pathogens (S. typhimurium and L. monocytogenes) on sprouts of broccoli than with the separate use of ClO2 and fumaric acid.
Kingsley et al. (2018) noted that ClO2 released from acidifying NaClO2 (1 mg) decreased the virus population on the inoculated blueberries more than 2.2 log CFU after 15 min; however, the high concentrations of ClO2 (≥ 10 mg acidified NaClO2) would damage their appearance and overall quality.
Toxic Substances Degradation
Many authors have shown that ClO2 can remove or significantly degrade pesticides, including insecticides and herbicides on fresh agricultural products and in water (Table 1), including diclofenac, deltamethrin (Wang et al. 2017) found that 50 mg L−1 of ClO2 solution could slow down ethylene production and increase soluble protein content and antioxidant activities in petals to extend the best view of tree peony cut flowers.
Condiments
Liu (2005) reported that a 5-min treatment with ClO2 solution of shallots and sliced ginger decreased the number of total colonies significantly. Similarly, Lian et al. (2007) found that treatment of low-salted garlic by 30 mg kg−1 ClO2 resulted in a decrease of the total numbers of colonies that were larger than that observed for other preservatives, SB (sodium benzoate) and PS (potassium sorbate). In addition, ClO2 inhibits the browning of garlic during storage as reported in recent studies (Lian et al. 2007).
Conclusions
ClO2 is extensively utilized commercially in several countries all over the world in order to inactivate microorganisms and maintain the quality of food. However, further work will be needed to develop new formulations and portable equipment able to detect actual pathogen concentrations, and to improve the use and application guidelines of ClO2 treatment for different agricultural products. In addition, intensive research is needed to understand the regulation mechanism of physiological and biochemical changes in genomics and proteomics of agricultural products after ClO2 treatment and the regulation of changes in appearance and colors as well as intrinsic nutrition is also required.
References
Adams, D. O., & Yang, S. F. (1979). Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proceeding of the National Academy of Science of United States of America, 76(1), 170–174. https://doi.org/10.1073/pnas.76.1.170.
Aieta, E. M., & Berg, J. D. (1986). A review of chlorine dioxide in drinking water treatment. Journal of American Water Works Association, 78(6), 62–72.
Artés, F., Gómez, P., Aguayo, E., Escalona, V., & Artés-Hernández, F. (2009). Sustainable sanitation techniques for kee** quality and safety of fresh-cut plant commodities. Postharvest Biology and Technology, 51(3), 287–296. https://doi.org/10.1016/j.postharvbio.2008.10.003.
Bai, X. L., Yang, S., Guo, K. X., Cheng, J., Liu, G. R., & **, R. Y. (2013). Study on bactericidal effect of chlorine dioxide gas on surface bacillus subtilis of bitter buckwheat. Cereals & Oils, 26(4), 29–31. https://doi.org/10.3969/j.issn.1008-9578.2013.04.010.
Barad, S., Horowitz, S. B., Kobiler, I., Sherman, A., & Prusky, D. (2014). Accumulation of the mycotoxin patulin in the presence of gluconic acid contributes to pathogenicity of Penicillium expansum. Molecular Plant-Microbe Interacttons, 27(1), 66–77. https://doi.org/10.1094/MPMI-05-13-0138-R.
Berg, J. D., Roberts, P. V., & Matin, A. (1986). Effect of chlorine dioxide on selected membrane functions of Escherichia-coli. Journal of Applied Bacteriology, 60(3), 213–220. https://doi.org/10.1111/j.1365-2672.1986.tb01075.x.
Bleecker, A. B., Estelle, M. A., Somerville, C., & Kende, H. (1988). Insensitivity to ethylene conferred by a dominant mutation in arabidopsis thaliana. Science, 241(4869), 1086–1089. https://doi.org/10.1126/science.241.4869.1086.
Boddie, R. L., Nickerson, S. C., & Adkinson, R. W. (2000). Efficacies of chlorine dioxide and lodophor teat dips during experimental challenge with Staphylococcus aureus and Streptococcus agalactiae. Journal of Dairy Science, 83(12), 2975–2979. https://doi.org/10.3168/jds.S0022-0302(00)75197-6.
Bull, R. J., Reckhow, D. A., Li, X., Humpage, A. R., Joll, C., & Hrudey, S. E. (2011). Potential carcinogenic hazards of non-regulated disinfection by-products: Haloquinones, halo-cyclopentene and cyclohexene derivatives, N-halamines, halonitriles, and heterocyclic amines. Toxicology, 286(1-3), 1–19. https://doi.org/10.1016/j.tox.2011.05.004.
Buschini, A., Carboni, P., Furlini, M., Poli, P., & Rossi, C. (2004). Sodium hypochlorite-, chlorine dioxide- and peracetic acid-induced genotoxicity detected by the Comet assay and Saccharomyces cerevisiae D7 tests. Mutagenesis, 19(2), 157–162. https://doi.org/10.1093/mutage/geh012.
Cao, L. K., Liu, M. Y., Zhang, H., Qian, B. J., Deng, Y., Song, X. Y., et al. (2010). Sanitizers affect chemical compositions and physical characteristics of few-flower wildrices under modified atmosphere packaging. Philippine Agricultural Scientist, 93(4), 446–453.
Chen, Q. Q., Wang, Y., Chen, F., Zhang, Y. Y., & Liao, X. J. (2014). Chlorine dioxide treatment for the removal of pesticide residues on fresh lettuce and in aqueous solution. Food Control, 40(1), 106–112. https://doi.org/10.1016/j.foodcont.2013.11.035.
Chen, X. L., Mei, G., & Ding, X. W. (2010). Effect of chlorine dioxide on the preservation of pork, cattle stomach and duck intestine. Science and Technology of Food Industry, 31(3), 342–345. https://doi.org/10.13386/j.issn1002-0306.2010.03.091.
Chen, Z., & Zhu, C. H. (2011). Combined effects of aqueous chlorine dioxide and ultrasonic treatments on postharvest storage quality of plum fruit (Prunus salicina L.). Postharvest Biology and Technology, 61(2), 117–123. https://doi.org/10.1016/j.postharvbio.2011.03.006.
Chen, Z., Zhu, C. H., & Han, Z. Q. (2011). Effects of aqueous chlorine dioxide treatment on nutritional components and shelf-life of mulberry fruit (Morus alba L.). Journal of Fermentation and Bioengineering, 111(6), 675–681. https://doi.org/10.1016/j.jbiosc.2011.01.010.
Chiabrando, V., Giuggioli, N., Maghenzani, M., Peano, C., & Giacalone, G. (2018). Improving storability of strawberries with gaseous chlorine dioxide in perforated clamshell packaging. Polish Journal of Food and Nutrition Sciences, 68(2), 141–148. https://doi.org/10.1515/pjfns-2017-0024.
Cho, J. L., Kim, C. K., Park, J., & Kim, J. (2017). Efficacy of aerosolized chlorine dioxide in reducing pathogenic bacteria on washed carrots. Food Science and Biotechnology, 26(4), 1129–1136. https://doi.org/10.1007/s10068-017-0139-6.
Chomkitichaia, W., Faiyueb, B., Rachtanapunc, P., Uthaibutraa, J., & Saengnila, K. (2014). Enhancement of the antioxidant defense system of post-harvested ‘Daw’ longan fruit by chlorine dioxide fumigation. Scientia Horticulturae, 178, 138–144. https://doi.org/10.1016/j.scienta.2014.08.016.
Chumyam, A., Shank, L., Uthaibutra, J., & Saengnil, K. (2016). Effects of chlorine dioxide on mitochondrial energy levels and redox status of ‘Daw’ longan pericarp during storage. Postharvest Biology and Technology, 116(2), 26–35. https://doi.org/10.1016/j.postharvbio.2016.01.002.
Chun, H. H., Kang, J. H., & Song, K. B. (2013a). Effects of aqueous chlorine dioxide treatment and cold storage on microbial growth and quality of blueberries. Journal of the Korean Society for Applied Biological Chemistry, 56(3), 309–315. https://doi.org/10.1007/s13765-013-3017-9.
Chun, H. H., & Song, K. B. (2014). Optimisation of the combined treatments of aqueous chlorine dioxide, fumaric acid and ultraviolet-C for improving the microbial quality and maintaining sensory quality of common buckwheat sprout. International Journal of Food Science and Technology, 49(1), 121–127. https://doi.org/10.1111/ijfs.12283.
Chun, H. H., Yu, D. J., & Song, K. B. (2013b). Effects of combined nonthermal treatment on microbial growth and the quality of minimally processed yam (Dioscorea japonica Thunb) during storage. International Journal of Food Science and Technology, 48(2), 334–340. https://doi.org/10.1111/j.1365-2621.2012.03191.x.
Cliffe-Byrnes, V., & O’Beirne, D. (2008). Effects of washing treatment on microbial and sensory quality of modified atmosphere (MA) packaged fresh sliced mushroom (Agaricus bisporus). Postharvest Biology and Technology, 48(2), 283–294. https://doi.org/10.1016/j.postharvbio.2007.10.012.
Deshwal, B. R., & Lee, H. K. (2004). Kinetics and mechanism of chloride based chlorine dioxide generation process from acidic sodium chlorate. Journal of Hazardous Materials, 108(3), 173–182. https://doi.org/10.1016/j.jhazmat.2003.12.006.
Deshwal, B. R., & Lee, H. K. (2005). Manufacture of chlorine dioxide from sodium chlorite: process chemistry. Journal of Industrial and Engineering Chemistry, 11(1), 125–136.
Du, J. H., Fu, M. R., Li, M. M., & **a, W. (2007). Effects of chlorine dioxide gas on postharvest physiology and storage quality of green bell pepper (Capsicum frutescens L. var. Longrum). Agricultural sciences in China, 6(2), 214–219. https://doi.org/10.1016/S1671-2927(07)60037-6.
Du, J. H., Fu, Y. C., & Wang, N. (2009). Effects of aqueous chlorine dioxide treatment on browning of fresh-cut lotus root. LWT - Food Science and Technology, 42(2), 654–659. https://doi.org/10.1016/j.lwt.2008.08.007.
Duan, Y. B., Zhao, F. L., Li, H., Zhou, Y. Y., Zhu, X. Y., Li, F. L., et al. (2016). Evaluation of aqueous chlorine dioxide for disinfecting plant explants. Vitro Cellular & Developmental Biology - Plant, 52(1), 38–44. https://doi.org/10.1007/s11627-015-9736-3.
FDA (2008). Code of Federal Regulations 21 CFR 173.300: Secondary direct food additives permitted in food for human consumption: Chlorine dioxide. [Revised as of April 1, 2003]. http://frwebgate5.access.gpo.gov/cgi-bin/waisgate.cgi?WAISdocID=201673176196+5+2+0&WAISaction=retrieve.
Feng, H., Yuan, Y., & Liu, L. J. (2010). Preparation of chlorine dioxide by catalytic method and its preservation effect on winter jujube. Journal of Anhui Agricultural Sciences, 38(30), 113–116. https://doi.org/10.3969/j.issn.0517-6611.2010.30.232.
Fu, M. R., & Du, J. H. (2004). The application of chlorine dioxide in food fresh-kee**. Food & Fermentation Industries, 113–116. https://doi.org/10.3321/j.issn:0253-990X.2004.08.028.
George, J. F. (2005). Chlorine dioxide offers seafood treatment alternative to aqueous chlorine. Global Aquaculture Advocate, 8(2), 36–37.
Gomez-Lopez, V. M. (2002). Some biochemical properties of polyphenol oxidase from two varieties of avocado. Food Chemistry, 8(2), 163–169. https://doi.org/10.1016/S0308-8146(01)00331-4.
Gómez-López, V. M., Devlieghere, F., Ragaert, P., & Debevere, J. (2007). Shelf-life extension of minimally processed carrots by gaseous chlorine dioxide. International Journal of Food Microbiology, 116(2), 221–227. https://doi.org/10.1016/j.ijfoodmicro.2006.12.008.
Gómez-López, V. M., Rajkovic, A., Ragaert, P., Smigic, N., & Devlieghere, F. (2009). Chlorine dioxide for minimally processed produce preservation: a review. Trends in Food Science & Technology, 20(1), 0–26. https://doi.org/10.1016/j.tifs.2008.09.005.
Gong, H. L., Wang, X. M., Yuan, H. J., & Feng, Z. P. (2011). Effects of chlorine dioxide on postharvest rots control and preservation of Lanzhou lily bulb. Transactions of the Chinese Society of Agricultural Engineering, 27(11), 359–364. https://doi.org/10.3969/j.issn.1002-6819.2011.11.067.
Guntiyaa, N., Bussabana, B., Faiyueb, B., Uthaibutraac, J., & Saengnilac, K. (2016). Application of gaseous chlorine dioxide for control of fungal fruit rot disease of harvested ‘Daw’ longan. Scientia Horticulturae, 213, 164–172. https://doi.org/10.1016/j.scienta.2016.10.019.
Guo, K. X., **, R. Y., Bai, X. L., Yang, S., & Liu, G. R. (2014a). Research on sterilization effect of chlorine dioxide gas on common buckwheat with bacterial contamination. Cereals & Oils, 27, 55–57. https://doi.org/10.3969/j.issn.1008-9578.2014.05.016.
Guo, Q., Wu, B., Peng, X., Wang, J., Li, Q., **, J., & Ha, Y. (2014b). Effects of chlorine dioxide treatment on respiration rate and ethylene synthesis of postharvest tomato fruit. Postharvest Biology and Technology, 93, 9–14. https://doi.org/10.1016/j.postharvbio.2014.01.013.
Han, G. D., Kwon, H., Kim, B. H., Kum, H. J., Kwon, K., & Kim, W. (2018). Effect of gaseous chlorine dioxide treatment on the quality of rice and wheat grain. Journal of Stored Products Research, 76, 66–70. https://doi.org/10.1016/j.jspr.2018.01.003.
Han, Y., Linton, R. H., Nielsen, S. S., & Nelson, P. E. (2001). Reduction of Listeria monocytogenes on green peppers (Capsicum annuum L.) by gaseous and aqueous chlorine dioxide and water washing and its growth at 7 °C. Journal of Food Protection, 64, 1730–1738. https://doi.org/10.4315/0362-028X-64.11.1730.
Hassenberg, K., Herppich, W. B., & Praeger, U. (2014). Chlorine dioxide for the reduction of human pathogens in lettuce washing process. Landtechnik, 69(4), 185–189. https://doi.org/10.15150/lt.2014.188.
Hu, Y. F., Li, N. N., Chen, J. R., Jia, Y. S., Liang, J., & Li, Y. F. (2016). Effect of chlorine dioxide on quality of giant salamander cutting meats in small modified atmosphere packaging. Advance Journal of Food Science and Technology, 10(4), 302–308. https://doi.org/10.19026/ajfst.10.2073.
Huang, J. L., Wang, L., Ren, N. Q., Ma, F., & Juli. (1997). Disinfection effect of chlorine dioxide on bacteria in water. Water Research, 31(3), 607–613. https://doi.org/10.1016/S0043-1354(96)00275-8.
Huang, X. H., Lu, R. H., Shi, M. N., Zhu, F. R., Huang, H. Y., & Luo, M. L. (2010). Application of chlorine dioxide on mulberry leaf disinfection. Guangxi Agricultural Sciences, 41(7), 714–718. https://doi.org/10.3969/j.issn.2095-1191.2010.07.023.
Huang, W. H., Cai, G. Q., Zhang, J. S., Liu, L. J., Lu., X. Y., Huang, H. X., et al. (2018). Efficiency and mechanism of removing chloral hydrate precursor by chlorine dioxide pre-oxidation. Acta Scientiae Circumstantiae, 38(4), 1514–1520. https://doi.org/10.13671/j.hjkxxb.2017.0424.
Hwang, E., Cash, J., & Zabik, M. J. (2001). Postharvest treatments for the reduction of mancozeb in fresh apples. Journal of Agricultural and Food Chemistry, 49(6), 3127–3132. https://doi.org/10.1021/jf010234h.
Islam, M. Z., Mele, M. A., Park, J. M., Kim, I. S., & Kang, H. M. (2017). Chlorine dioxide gas retain postharvest quality and shelf life of tomato during modified atmosphere packaging storage. Agrivita: Journal of Agricultural Science, 39(3), 233–238. https://doi.org/10.17503/agrivita.v39i3.1454.
Ji, X. T., Nian, Y. Y., Xue, P., Wang, L., Cui, Y. T., & Sun, L. M. (2018). Effect of chlorine dioxide on pomfret quality during superchilled storage. Meat Research, 32(2), 60–65, 69. https://doi.org/10.7506/rlyj1001-8123-201802008.
Jiang, L. Q., Feng, W. Y., Li, F., Xu, J. Y., Ma, Y. P., & Ma, H. L. (2015). Effect of one-methylcyclopropene (1-MCP) and chlorine dioxide (ClO2) on preservation of green walnut fruit and kernel traits. Journal of Food Science and Technology, 52(1), 267–275. https://doi.org/10.1007/s13197-013-0996-9.
Kim, H. G., & Song, K. B. (2017). Combined treatment with chlorine dioxide gas, fumaric acid, and ultraviolet-C light for inactivating Escherichia coli O 157:H 7 and Listeria monocytogenes inoculated on plums. Food Control, 71, 371–375. https://doi.org/10.1016/j.foodcont.2016.07.022.
Kim, J. M., Huang, T. S., Marshall, M. R., & Wei, C. I. (1999). Chlorine dioxide treatment of seafoods to reduce bacterial loads. Journal of Food Science, 64(6), 1089–1093. https://doi.org/10.1111/j.1365-2621.1999.tb12288.x.
Kim, Y. J., Kim, M. H., & Song, K. B. (2009). Efficacy of aqueous chlorine dioxide and fumaric acid for inactivating pre-existing microorganisms and Escherichia coli O 157:H7, Salmonella typhimurium, and Listeria monocytogenes on broccoli sprouts. Food Control, 20(11), 1002–1005. https://doi.org/10.1016/j.foodcont.2008.12.005.
Kingsley, D. H., Pérez-Pérez, R. E., Niemira, B. A., & Fan, X. (2018). Evaluation of gaseous chlorine dioxide for the inactivation of Tulane virus on blueberries. International Journal of Food Microbiology, 273, 28–32. https://doi.org/10.1016/j.ijfoodmicro.2018.01.024.
Ku, K. J., Ma, Y. H., Shin, H. Y., & Lee, S. H. (2006). Effects of chlorine dioxide treatment on quality and microbial change of Agaricus bisporus Sing during storage. Journal of the Korean Society of Food Science and Nutrition, 35(7), 955–959. https://doi.org/10.3746/jkfn.2006.35.7.955.
Lai, J. L., Li, C. X., Weng, S. J., & Wei, M. K. (2015). Effects of composite application of chitonsan and chlorine dioxide on bayberry preserving. Hubei Agricultural Sciences, 54(13), 3213–3217. https://doi.org/10.14088/j.cnki.issn0439-8114.2015.13.037.
Lee, S. H., Shin, H. Y., Ku, K. J., **, Y. Y., Jeon, S. J., Chae, H. S., et al. (2007). Quality change of red meat by chlorine dioxide treatment during storage. Korean Journal of Food Science and Technology, 39(2), 222–227.
Legay, C., Rodriguez, M. J., Sérodes, J. B., & Levallois, P. (2010). Estimation of chlorination by-products presence in drinking water in epidemiological studies on adverse reproductive outcomes: a review. The Science of the Total Environment, 408(3), 456–472. https://doi.org/10.1016/j.scitotenv.2009.10.047.
Li, J. R., **, R. Y., & Cheng, S. Y. (2012a). Research status and application of solid chlorine dioxide. Chenmical Intermediate, 9(1), 44–47. https://doi.org/10.3969/j.issn.1672-8114.2012.01.010.
Li, M., Tian, S. L., Cheng, J. X., Tian, C. J., Mu, Y. W., & Song, J. (2017a). Inhibitory effect of chlorine dioxide on the spout of potato tubers. Gansu Agricultural Science and Technology, 2, 33–36. https://doi.org/10.3969/j.issn.1001-1463.2017.02.010.
Li, M., Tian, S. L., **e, M. H., Li, S. Q., Zhang, X., Cheng, J. X., et al. (2012b). Suppression of ClO2 treatment on browning and the analysis of browning mechanism of Agaricus bisporus. Acta Botanica Boreali-Occidentalia Sinica, 32(8), 1621–1625. https://doi.org/10.3969/j.issn.1001-4025.2012.08.018.
Li, S. X., Li, Z. J., Zhu, L. Y., & Zhao, D. J. (2017b). Study on degradation of cylindrospermopsin by chlorine dioxide. Environmental Science and Technology, 40(1), 60–63. https://doi.org/10.3969/j.issn.1003-6504.2017.01.011.
Li, Z. L., Zhang, D. Y., Wang, Y. F., Fu, S. S., Xue, P. Z., & Dong, Y. (2014). The development of the new type of high purity chlorine dioxide generator. Shandong Chemical Industry, 43, 26–27. https://doi.org/10.19319/j.cnki.issn.1008-021x.2014.03.011.
Lian, Y., Li, Y., Zuo, J. H., & Wang, H. M. (2007). Research on the processing technology and storability of low-salted garlic. China Condiment, 5, 48–51. https://doi.org/10.3969/j.issn.1000-9973.2007.05.011.
Liu, M., Qian, B., Zhang, H., Deng, Y., Shen, Y., **, J., & Cao, L. (2010). Sanitizer treatments alleviate lignification of sliced few-flower wildrice (Zizania latifolia Turcz.). Food Research International, 43(10), 2363–2368. https://doi.org/10.1016/j.foodres.2010.09.004.
Liu, Q. (2005). Bacterial effect of chlorine dioxide in food. Journal of Liaoning Chemical Industry, 34, 29–30. https://doi.org/10.3969/j.issn.1004-0935.2005.01.010.
Liu, X. L., & L, Y., Wu, M. S., Liu, H., & Hong, H. A. (2014). Effect of chlorine dioxide on degradation of deltamethrin in water. Bulletin of Agricultural Science and Technology, 9, 138–141. https://doi.org/10.3969/j.issn.1000-6400.2014.09.048.
Luo, Z. S., Huang, H., Wang, X., Li, L., & Ru, Q. M. (2017). Effect of solid chlorine dioxide on quality preservation of penaeus vannamei. Modern Food Science and Technology, 33(5), 148–154. https://doi.org/10.13982/j.mfst.1673-9078.2017.5.024.
Mahmoud, B. S. M., Bhagat, A. R., & Linton, R. H. (2007). Inactivation kinetics of inoculated Escherichia coli O 157:H7 and Salmonella enterica on strawberries by chlorine dioxide gas. Food Microbiology, 24, 736–744. https://doi.org/10.1016/j.fm.2007.03.006 7-8.
Millan-Sangoa, D., Sammuta, E., Impeb, J. F. V., & Valdramidisa, V. P. (2017). Decontamination of alfalfa and mung bean sprouts by ultrasound and aqueous chlorine dioxide. LWT - Food Science and Technology, 78, 90–96. https://doi.org/10.1016/j.lwt.2016.12.015.
Nian, L. K., Meng, H. Y., Su, X. L., & Shi, G. A. (2017). Effect of chlorine dioxide addition to vase solution on fresh-kee** of three peony cut flowers. Journal of Plant Physiology, 11(53), 2022–2030. https://doi.org/10.13592/j.cnki.ppj.2017.0248.
Ölmez, H., & Kretzschmar, U. (2009). Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT - Food Science and Technology, 42(3), 686–693. https://doi.org/10.1016/j.lwt.2008.08.001.
Park, S., Beuchat, L. R., Kim, H., & Ryu, J. H. (2017). Inactivation of Salmonella enterica in chicken feces on the surface of eggshells by simultaneous treatments with gaseous chlorine dioxide and mild wet heat. Food Microbiology, 62, 202–206. https://doi.org/10.1016/j.fm.2016.10.026.
Park, S. H., & Kang, D. H. (2015). Antimicrobial effect of chlorine dioxide gas against foodborne pathogens under differing conditions of relative humidity. LWT - Food Science and Technology, 60(1), 186–191. https://doi.org/10.1016/j.lwt.2014.09.031.
Park, S. H., Kim, W. J., & Kang, D. H. (2018a). Effect of relative humidity on inactivation of foodborne pathogens using chlorine dioxide gas and its residues on tomatoes. Letters in Applied Microbiology, 67(2), 154–160. https://doi.org/10.1111/lam.13002.
Park, S. Y., Jung, S. J., & Ha, S. D. (2018b). Synergistic effects of combined X-ray and aqueous chlorine dioxide treatments against Salmonella Typhimurium biofilm on quail egg shells. LWT - Food Science and Technology, 92, 54–60. https://doi.org/10.1016/j.lwt.2018.02.010.
Praeger, U., Herppich, W. B., & Hassenberg, K. (2016). Aqueous chlorine dioxide treatment of horticultural produce: effects on microbial safety and produce quality-a review. Critical Reviews in Food Science and Nutrition, 58(2), 318–333. https://doi.org/10.1080/10408398.2016.1169157.
Qu, Q. L., Yang, X. Y., Chen, Q. M., Zhang, X. H., & Fu, M. R. (2016). Effect of 1-MCP, ClO2 and NaHSO3 on lipid oxidation and antioxidant activities of green walnut kernel. Science and Technology of Food Industry, 37(20), 336–340 https://doi.org/11.1759.TS.20160704.0910.054.
Ramos, B., Miller, F. A., Brandão, T. R. S., Teixeira, P., & Silva, C. L. M. (2013). Fresh fruits and vegetables—an overview on applied methodologies to improve its quality and safety. Innovative Food Science and Emerging Technologies, 20, 1–15. https://doi.org/10.1016/j.ifset.2013.07.002.
Remorini, D., Landi, M., Tardelli, F., Lugani, A., Massai, R., Graziani, G., Fogliano, V., & Guidi, L. (2015). Effect of chlorine dioxide and ascorbic acid on enzymatic browning and shelf life of fresh-cut red delicious and granny smith apples. Journal of Food Processing and Preservation, 39(6), 2925–2934. https://doi.org/10.1111/jfpp.12544.
Saade, C., Annous, B. A., Gualtieri, A. J., Schaich, K. M., Liu, L., & Yam, K. L. (2017). System feasibility: designing a chlorine dioxide self-generating package label to improve fresh produce safety part I: extrusion approach. Innovative Food Science and Emerging Technologies, 43, 102–111. https://doi.org/10.1016/j.ifset.2017.08.004.
Saade, C., Annous, B. A., Gualtieri, A. J., Schaich, K. M., Liu, L., & Yam, K. L. (2018). System feasibility: designing a chlorine dioxide self-generating package label to improve fresh produce safety part II: solution casting approach. Innovative Food Science and Emerging Technologies, 47, 110–119. https://doi.org/10.1016/j.ifset.2018.02.003.
Saengnil, K., Chumyam, A., Faiyue, B., & Uthaibutra, J. (2014). Use of chlorine dioxide fumigation to alleviate enzymatic browning of harvested ‘Daw’ longan pericarp during storage under ambient conditions. Postharvest Biology and Technology, 91, 49–56. https://doi.org/10.1016/j.postharvbio.2013.12.016.
Shan, X. M., & Lu, J. C. (2013). Advances on the determination of chlorine dioxide in water. Pollution Control Technology, 26(4), 60–65.
Sharma, V. K., & Sohn, M. (2012). Reactivity of chlorine dioxide with amino acids, peptides, and proteins. Environmental Chemistry Letters, 10(3), 255–264. https://doi.org/10.1007/s10311-012-0355-5.
Shen, Y. L., & Yan, X. M. (2001). Application of stable chlorine dioxide in disinfection. Journal of Chengde Petroleum College, 3(3), 21–24. https://doi.org/10.3969/j.issn.1008-9446.2001.03.007.
Shin, J. H., Chang, S., & Kang, D. H. (2004). Application of antimicrobial ice for reduction of foodborne pathogens (Escherichia coli O 157 :H 7 , Salmonella Typhimurium, Listeria monocytogenes) on the surface of fish. Jourmal of Applied Microbiology, 97(5), 916–922. https://doi.org/10.1111/j.1365-2672.2004.02343.x.
Shirasaki, Y., Matsuura, A., Uekusa, M., Ito, Y., & Hayashi, T. (2016). A study of the properties of chlorine dioxide gas as a fumigant. Experimental Animals, 65(3), 303–310. https://doi.org/10.1538/expanim.15-0092.
Simonet, J., & Gantzer, C. (2006). Degradation of the Poliovirus 1 genome by chlorine dioxide. Journal of Applied Microbiology, 100(4), 862–870. https://doi.org/10.1111/j.1365-2672.2005.02850.x.
Smith, D. J., Ernst, W., & Herges, G. R. (2015). Chloroxyanion residues in cantaloupe and tomatoes after chlorine dioxide gas sanitation. Journal of Agricultural and Food Chemistry, 63(43), 9640–9649. https://doi.org/10.1021/acs.jafc.5b04153.
Sun, C., Zhu, P., Ji, J., Sun, J., Tang, L., Pi, F., & Sun, X. (2017a). Role of aqueous chlorine dioxide in controlling the growth of Fusarium graminearum and its application on contaminated wheat. LWT - Food Science and Technology, 84, 555–561. https://doi.org/10.1016/j.lwt.2017.03.032.
Sun, X. X., Baldwin, E., Ference, C., Narciso, J., Plotto, A., Ritenour, M., et al. (2017b). The effect of controlled-release chlorine dioxide on the preservation of grapefruit. Hort Science, 52(1), 122–126. https://doi.org/10.21273/hortsci11363-16.
Sun, X. X., Elizabeth, B., & Bai, J. H. (2018). Applications of gaseous chlorine dioxide on postharvest handling and storage of fruits and vegetables – a review. Food Control, 95, 18–26. https://doi.org/10.1016/j.foodcont.2018.07.044.
Sun, X. X., Zhou, B., Luo, Y. G., Ference, C., Baldwin, E., Harrison, K., et al. (2017c). Effect of controlled-release chlorine dioxide on the quality and safety of cherry/grape tomatoes. Food Control, 82, 26–30. https://doi.org/10.1016/j.foodcont.2017.06.021.
Swanson, S. P., Helaszek, C., Buck, W. B., Rood, H. D., & Haschek, W. M. (1988). The role of intestinal microflora in the metabolism of trichothecene mycotoxins. Food and Chemisry Toxicology, 26(10), 823–829. https://doi.org/10.1016/0278-6915(88)90021-X.
Tan, L. (2018). Research and development trend of chlorine dioxide preparation. Chemical Engineering Design Communications, 44(08), 71. https://doi.org/10.3969/j.issn.1003-6490.2018.08.065.
Tian, F. X., Xu, B., Zhang, T. Y., & Gao, N. Y. (2014). Degradation of phenylurea herbicides by chlorine dioxide and formation of disinfection by-products during subsequent chlor(am)ination. Chemical Engineering Journal, 258, 210–217. https://doi.org/10.1016/j.cej.2014.07.094.
Vandekinderen, I., Devlieghere, F., Van Camp, J., Kerkaert, B., Cucu, T., Ragaert, P., et al. (2009). Effects of food composition on the inactivation of foodborne microorganisms by chlorine dioxide. International Journal of Food Microbiology, 131(2-3), 138–144. https://doi.org/10.1016/j.ijfoodmicro.2009.02.004.
Visvalingam, J., & Holley, R. A. (2018). Evaluation of chlorine dioxide, acidified sodium chlorite and peroxyacetic acid for control of Escherichia coli O 157 :H 7 in beef patties from treated beef trim. Food Research International, 103, 295–300. https://doi.org/10.1016/j.foodres.2017.10.051.
Wang, J. L., & Guo, G. Q. (2001). Latest advances on the technology of preperation and application of chlorine dioxide (I). Shanghai Chemical Industry, 2, 20–21. https://doi.org/10.16759/j.cnki.issn.1004-017x.2001.02.004.
Wang, H. L., & Hu, S. Q. (2008). Application of chlorine dioxide gas and exploitation of the generator. Shanxi Chemical Industry, 28(1), 41–42. https://doi.org/10.16525/j.cnki.cn14-1109/tq.2008.01.010.
Wang, D., Qin, W., Yu, Z. H., Zhong, J. C., **ang, Y. H., Wang, J. Y., et al. (2002). Study on antistaling action of chlorine dioxide for raw milk. China Dairy Industry, 30(5), 47–48. https://doi.org/10.3969/j.issn.1001-2230.2002.05.014.
Wang, D. P., Zhang, D. D., Chen, W. Y., Yu, S. J., & Shi, X. M. (2010a). Retention of vibrio parahaemolyticus in oyster tissues after chlorine dioxide treatment. International Journal of Food Microbiology, 137(1), 76–80. https://doi.org/10.1016/j.ijfoodmicro.2009.10.022.
Wang, X., Chen, Q. Q., Zhang, Y. Y., Chen, F., & Li, S. (2010b). Research on the degradation of dimethoate and malathion with chlorine dioxide treatment. Food Science and Technology, 35(12), 291–295. https://doi.org/10.13684/j.cnki.spkj.2010.12.055.
Wang, Y. L., Liu, H. J., Liu, G. G., & **e, Y. H. (2014). Oxidation of diclofenac by aqueous chlorine dioxide: Identification of major disinfection byproducts and toxicity evaluation. Science of the Total Environment, 473-474, 437–445. https://doi.org/10.1016/j.scitotenv.2013.12.056.
Wei, J., Chen, Y., Tiemur, A., Wang, J., & Wu, B. (2018). Degradation of pesticide residues by gaseous chlorine dioxide on table grapes. Postharvest Biology and Technology, 137, 142–148. https://doi.org/10.1016/j.postharvbio.2017.12.001.
Wen, G., Xu, X., Huang, T., Zhu, H., & Ma, J. (2017). Inactivation of three genera of dominant fungal spores in groundwater using chlorine dioxide: effectiveness, influencing factors, and mechanisms. Water Research, 125, 132–140. https://doi.org/10.1016/j.watres.2017.08.038.
WHO (2000). Environmental health criteria 216, disinfectants and disinfectant by-products. http://www.inchem.org/documents/ehc/ehc/ehc216.htm. Accessed 9 Oct 2018.
Wilson, S. C., Brasel, T. L., Martin, J. M., Wu, C., Andriychuk, L., Douglas, D. R., Cobos, L., & Straus, D. C. (2005). Efficacy of chlorine dioxide as a gas and in solution in the inactivation of two trichothecene mycotoxins. International Journal of Toxicology, 24(3), 181–186. https://doi.org/10.1080/10915810590953437.
Wu, B., Guo, Q., Wang, G., Peng, X., Wang, J., & Che, F. (2015). Effects of different postharvest treatments on the physiology and quality of ‘**aobai’ apricots at room temperature. Journal of Food Science and Technology, 52(4), 2247–2255. https://doi.org/10.1007/s13197-014-1288-8.
Wu, B., Zhong, M., Wang, Z. R., & Wang, J. D. (2010). Controlled gas release and application of solid-state chlorine dioxide preservative. Food Science, 31(8), 294–296.
Wu, V. C. H., & Rioux, A. (2010). A simple instrument-free gaseous chlorine dioxide method for microbial decontamination of potatoes during storage. Food Microbiology, 27(1), 179–184. https://doi.org/10.1016/j.fm.2009.08.007.
**ao, L. M., Zhong, M., Wu, B., Guo, F. Y., & Wang, J. D. (2009). Effects of 1-methylcyclopropene and chlorine dioxide on preservation of **njiang flat peaches. Food Science, 30(12), 276–280. https://doi.org/10.3321/j.issn1002-6630.2009.12.063.
Xu, F. X., Wang, S. H., Xu, J., Liu, S. Y., & Li, G. D. (2016). Effects of combined aqueous chlorine dioxide and UV-C on shelf-life quality of blueberries. Postharvest Biology and Technology, 117, 125–131. https://doi.org/10.1016/j.postharvbio.2016.01.012.
Yang, H. Q., Zheng, J. Y., Huang, C. Q., Zhao, X. F., Chen, H. Y., & Sun, Z. D. (2015). Effects of combined aqueous chlorine dioxide and chitosan coatings on microbial growth and quality maintenance of fresh-cut bamboo shoots (Phyllostachys praecox f. prevernalis.) during storage. Food and Bioprocess Technology, 8(5), 1011–1019. https://doi.org/10.1007/s11947-014-1463-y.
Yang, X. Y., Zhang, X. H., Fu, M. R., Chen, Q. M., & Muzammil, J. M. (2018). Chlorine dioxide fumigation generated by a solid releasing agent enhanced the efficiency of 1-MCP treatment on the storage quality of strawberry. Journal of Food Science and Technology, 1-8. https://doi.org/10.1007/s13197-018-3114-1.
Yaron, S., & Römling, U. (2014). Biofilm formation by enteric pathogens and its role in plant colonization and persistence. Microbial Biotechnology, 7(6), 496–516. https://doi.org/10.1111/1751-7915.12186.
Zhang, X. M., & Fu, M. R. (2018). Inhibitory effect of chlorine dioxide (ClO2) fumigation on growth and patulin production and its mechanism in Penicillum expansum. LWT - Food Science and Technology, 96, 335–343. https://doi.org/10.1016/j.lwt.2018.05.051.
Zhang, X. M., Fu, M. R., & Chen, Q. M. (2018a). Effect of chlorine dioxide (ClO2) on patulin produced by Penicillum expansum and involved mechanism. Journal of the Science of Food and Agriculture, 99(4), 1961–1968. https://doi.org/10.1002/jsfa.9394.
Zhang, X. M., Li, J. P., Fu, M. R., **, T., Yang, X. Y., Han, C., et al. (2018b). Effects of the combination of 1-MCP and ClO2 on shelf life quality of green pepper. Science and Technology of Food Industry, 1–8. https://doi.org/10.13386/j.issn1002-0306.2018.13.050.
Zhang, Y. M., & Zhang, K. C. (2016). Study on the color of the lotus root sterilization of fresh cut chlorine dioxide. Farm Products Processing, 8, 19–20. https://doi.org/10.16693/j.cnki.1671-9646(X).2016.08.006.
Zhao, W. C., & Long, D. X. (2014). Effect of chlorine dioxide oxidation on degradation of methamidophs in wastewater. Journal of Environmental Hygiene, 4(1), 65–68. https://doi.org/10.16693/j.cnki.1671-9646(X).2016.08.006.
Zhou, S., Hu, C., Zhao, G., Liu, L., Sheen, S., & Yam, K. L. (2018). A novel gaseous chlorine dioxide generating method utilizing carbon dioxide and moisture respired from tomato for Salmonella inactivation. Food Control, 89, 54–61. https://doi.org/10.1016/j.foodcont.2018.01.009.
Funding
This research project was financially supported by the National Science Foundation of China (Project No. 31871854).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ran, Y., Qingmin, C. & Maorun, F. Chlorine Dioxide Generation Method and Its Action Mechanism for Removing Harmful Substances and Maintaining Quality Attributes of Agricultural Products. Food Bioprocess Technol 12, 1110–1122 (2019). https://doi.org/10.1007/s11947-019-02279-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-019-02279-x