Abstract
Purpose of Review
We highlight the clinical development of Poly (ADP-Ribose) polymerase (PARP) inhibitors in prostate cancer.
Recent Findings
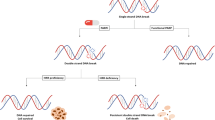
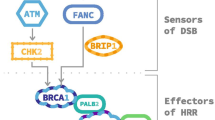
Approximately 10 to 30% of metastatic prostate cancer patients carry germline or somatic mutations in DNA repair pathways. BRCA2 is the most commonly mutated gene in DNA damage repair pathways. Because of its critical function in homologous recombination repair (HRR) machinery, deleterious BRCA2 mutation enables synthetic lethality to a PARP inhibitor. Olaparib demonstrated clinical benefit in patients with deleterious mutations in HRR-related genes and most clearly in patients with BRCA2 mutations. Olaparib received the US FDA approval or mCRPC patients with a qualifying HRR gene mutation in May 2020. Rucaparib received an accelerated FDA approval for patients with BRCA1- or BRCA2-mutated mCRPC based on 43% objective response rate in a phase II study. To expand the application of a PARP inhibitor, several trials have evaluated various combination strategies with an androgen receptor signaling inhibitor, immunotherapy, radium-223, and others. While no PARP inhibitor combination regimen has been approved, promising data from a PARP inhibitor and an ASI combination have been reported.
Summary
PARP inhibitor represents a standard treatment for patient with mCRPC with germline or somatic mutations in BRCA2 and other HRR pathway genes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Saad F, Efstathiou E, Attard G, Flaig TW, Franke F, Goodman OB Jr, Oudard S, Steuber T, Suzuki H, Wu D, Yeruva K, De Porre P, Brookman-May S, Li S, Li J, Thomas S, Bevans KB, Mundle SD, McCarthy SA, Rathkopf DE. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021;22(11):1541–59. https://doi.org/10.1016/s1470-2045(21)00402-2.
• Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, Garofalo A, Gulati R, Carreira S, Eeles R, Elemento O, Rubin MA, Robinson D, Lonigro R, Hussain M, Chinnaiyan A, Vinson J, Filipenko J, Garraway L, Taplin ME, AlDubayan S, Han GC, Beightol M, Morrissey C, Nghiem B, Cheng HH, Montgomery B, Walsh T, Casadei S, Berger M, Zhang L, Zehir A, Vijai J, Scher HI, Sawyers C, Schultz N, Kantoff PW, Solit D, Robson M, Van Allen EM, Offit K, de Bono J, Nelson PS. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–53. https://doi.org/10.1056/NEJMoa1603144.PubMedPMID:27433846;PMCID:4986616. This work was the report of germline sequencing of metastatic prostate cancer patients showing that 12% of metastatic prostate cancer patients carry germline DNA-repair gene mutations. The frequencies of these germline mutations among men with metastatic disease did not differ significantly according to age at diagnosis or family history of prostate cancer. This work provided a rationale for a NCCN guideline recommendation of routine germline sequencing in patients with metastatic prostate cancer.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H, Abida W, Bradley RK, Vinson J, Cao X, Vats P, Kunju LP, Hussain M, Feng FY, Tomlins SA, Cooney KA, Smith DC, Brennan C, Siddiqui J, Mehra R, Chen Y, Rathkopf DE, Morris MJ, Solomon SB, Durack JC, Reuter VE, Gopalan A, Gao J, Loda M, Lis RT, Bowden M, Balk SP, Gaviola G, Sougnez C, Gupta M, Yu EY, Mostaghel EA, Cheng HH, Mulcahy H, True LD, Plymate SR, Dvinge H, Ferraldeschi R, Flohr P, Miranda S, Zafeiriou Z, Tunariu N, Mateo J, Perez-Lopez R, Demichelis F, Robinson BD, Schiffman M, Nanus DM, Tagawa ST, Sigaras A, Eng KW, Elemento O, Sboner A, Heath EI, Scher HI, Pienta KJ, Kantoff P, de Bono JS, Rubin MA, Nelson PS, Garraway LA, Sawyers CL, Chinnaiyan AM. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–28. https://doi.org/10.1016/j.cell.2015.05.001.PubMedPMID:26000489;PMCID:4484602.
Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, Barron D, Danila D, Rathkopf D, Morris M, Slovin S, McLaughlin B, Curtis K, Hyman DM, Durack JC, Solomon SB, Arcila ME, Zehir A, Syed A, Gao J, Chakravarty D, Vargas HA, Robson ME, Joseph V, Offit K, Donoghue MTA, Abeshouse AA, Kundra R, Heins ZJ, Penson AV, Harris C, Taylor BS, Ladanyi M, Mandelker D, Zhang L, Reuter VE, Kantoff PW, Solit DB, Berger MF, Sawyers CL, Schultz N, Scher HI. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol. 2017;2017. https://doi.org/10.1200/PO.17.00029.
Daniels CM, Ong SE, Leung AK. The promise of proteomics for the study of ADP-ribosylation. Mol Cell. 2015;58(6):911–24. https://doi.org/10.1016/j.molcel.2015.06.012.
Vyas S, Chesarone-Cataldo M, Todorova T, Huang YH, Chang P. A systematic analysis of the PARP protein family identifies new functions critical for cell physiology. Nat Commun. 2013;4:2240. https://doi.org/10.1038/ncomms3240.
Sousa FG, Matuo R, Soares DG, Escargueil AE, Henriques JAP, Larsen AK, Saffi J. PARPs and the DNA damage response. Carcinogenesis. 2012;33(8):1433–40. https://doi.org/10.1093/carcin/bgs132.
Kawamitsu H, Miwa M, Tanaka Y, Sakamoto H, Terada M, Hoshi A, Sugimura T. Inhibitors of poly(adenosine diphosphate ribose) polymerase potentiate the antitumor activity of bleomycin against Ehrlich ascites carcinoma. J Pharmacobiodyn. 1982;5(11):900–4. https://doi.org/10.1248/bpb1978.5.900.
Park SD, Kim CG, Kim MG. Inhibitors of poly(ADP-ribose) polymerase enhance DNA strand breaks, excision repair, and sister chromatid exchanges induced by alkylating agents. Environ Mutagen. 1983;5(4):515–25. https://doi.org/10.1002/em.2860050402.
Plummer R, Jones C, Middleton M, Wilson R, Evans J, Olsen A, Curtin N, Boddy A, McHugh P, Newell D, Harris A, Johnson P, Steinfeldt H, Dewji R, Wang D, Robson L, Calvert H. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res. 2008;14(23):7917–23. https://doi.org/10.1158/1078-0432.CCR-08-1223.
Calabrese CR, Almassy R, Barton S, Batey MA, Calvert AH, Canan-Koch S, Durkacz BW, Hostomsky Z, Kumpf RA, Kyle S, Li J, Maegley K, Newell DR, Notarianni E, Stratford IJ, Skalitzky D, Thomas HD, Wang LZ, Webber SE, Williams KJ, Curtin NJ. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 2004;96(1):56–67. https://doi.org/10.1093/jnci/djh005.
Ben-Hur E, Utsumi H, Elkind MM. Inhibitors of poly (ADP-ribose) synthesis enhance radiation response by differentially affecting repair of potentially lethal versus sublethal damage. Br J Cancer Suppl. 1984;6:39–42.
• Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. https://doi.org/10.1038/nature03445. This study, in concordance with the paper published by Bryant et al. in the same issue of Nature in 2005, demonstrated the synergistic effect PARP1 depletion and BRCA deficiency have on BRCA-1 and BRCA-2 KO stem cells, laying the groundwork for the concept known as synthetic lethality.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. https://doi.org/10.1038/nature03443.
• Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. https://doi.org/10.1056/NEJMoa0900212. This was the first report of a PARP inhibitor trial that provided clinical evidence of its synthetic lethality in germline BRCA mutation carriers. Radiologic or tumor marker response was observed in patients with breast, ovarian, and prostate cancers foreshadowing the efficacy in future studies.
Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, Ji J, Takeda S, Pommier Y. Trap** of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72(21):5588–99. https://doi.org/10.1158/0008-5472.CAN-12-2753.
Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, Morris J, Teicher B, Doroshow JH, Pommier Y. Stereospecific PARP trap** by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014;13(2):433–43. https://doi.org/10.1158/1535-7163.MCT-13-0803.
Hopkins TA, Ainsworth WB, Ellis PA, Donawho CK, DiGiammarino EL, Panchal SC, Abraham VC, Algire MA, Shi Y, Olson AM, Johnson EF, Wilsbacher JL, Maag D. PARP1 trap** by PARP inhibitors drives cytotoxicity in both cancer cells and healthy bone marrow. Mol Cancer Res. 2019;17(2):409–19. https://doi.org/10.1158/1541-7786.MCR-18-0138.
• de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Mehra N, Goessl C, Kang J, Burgents J, Wu W, Kohlmann A, Adelman CA, Hussain M. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–102. https://doi.org/10.1056/NEJMoa1911440. This is the report of phase III trial of olaparib versus a second-line ASI demonstrating the improvement of rPFS in patients with BRCA1, BRCA2, or ATM mutations (cohort A), which was the primary analysis cohort. Analysis of combined cohorts A and B which included mutations in 11 other DNA repair genes continued to show a significant improvement in rPFS over a second-line ASI.
Abida W, Patnaik A, Campbell D, Shapiro J, Bryce AH, McDermott R, Sautois B, Vogelzang NJ, Bambury RM, Voog E, Zhang J, Piulats JM, Ryan CJ, Merseburger AS, Daugaard G, Heidenreich A, Fizazi K, Higano CS, Krieger LE, Sternberg CN, Watkins SP, Despain D, Simmons AD, Loehr A, Dowson M, Golsorkhi T, Chowdhury S, TRITON2 investigators. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38(32):3763–72. https://doi.org/10.1200/JCO.20.01035.
de Bono JS, Mehra N, Scagliotti GV, Castro E, Dorff T, Stirling A, Stenzl A, Fleming MT, Higano CS, Saad F, Buttigliero C, van Oort IM, Laird AD, Mata M, Chen HC, Healy CG, Czibere A, Fizazi K. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021;22(9):1250–64. https://doi.org/10.1016/S1470-2045(21)00376-4.
Smith MR, Fizazi K, Sandhu SK, Kelly WK, Efstathiou E, Lara P, Yu EY, George DJ, Chi KN, Saad F, Summa J, Freedman JM, Mason G, Espina BM, Zhu E, Ricci DS, Snyder LA, Simon JS, Cheng S, Scher HI. Niraparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD): correlative measures of tumor response in phase II GALAHAD study. J Clin Oncol. 2020;38(6_suppl):118. https://doi.org/10.1200/JCO.2020.38.6_suppl.118.
• Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A’Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–708. https://doi.org/10.1056/NEJMoa1506859. This work is the first report of a phase II PARP inhibitor trial in mCRPC showing 88% response rate among patients with DNA repair defect. This work laid the foundation for biomarker development of PARP inhibitors in mCRPC and paved the way for the pivotal phase III trial.
Mateo J, Porta N, Bianchini D, McGovern U, Elliott T, Jones R, Syndikus I, Ralph C, Jain S, Varughese M, Parikh O, Crabb S, Robinson A, McLaren D, Birtle A, Tanguay J, Miranda S, Figueiredo I, Seed G, Bertan C, Flohr P, Ebbs B, Rescigno P, Fowler G, Ferreira A, Riisnaes R, Pereira R, Curcean A, Chandler R, Clarke M, Gurel B, Crespo M, Nava Rodrigues D, Sandhu S, Espinasse A, Chatfield P, Tunariu N, Yuan W, Hall E, Carreira S, de Bono JS. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):162–74. https://doi.org/10.1016/S1470-2045(19)30684-9.
Hussain M, Daignault-Newton S, Twardowski PW, Albany C, Stein MN, Kunju LP, Siddiqui J, Wu YM, Robinson D, Lonigro RJ, Cao X, Tomlins SA, Mehra R, Cooney KA, Montgomery B, Antonarakis ES, Shevrin DH, Corn PG, Whang YE, Smith DC, Caram MV, Knudsen KE, Stadler WM, Feng FY, Chinnaiyan AM. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J Clin Oncol. 2018;36(10):991–9. https://doi.org/10.1200/jco.2017.75.7310.
Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Roubaud G, Ozguroglu M, Kang J, Burgents J, Gresty C, Corcoran C, Adelman CA, de Bono J, Investigators PRT. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383(24):2345–57. https://doi.org/10.1056/NEJMoa2022485.
Agency EM. An overview of Lynparza and why it is authorised in the EU: European Medicines Agency; 2020 [updated 11/2020; cited 2021 9/12/2021]. Available from: https://www.ema.europa.eu/en/documents/overview/lynparza-epar-medicine-overview_en.pdf.
Abida W, Campbell D, Patnaik A, Shapiro JD, Sautois B, Vogelzang NJ, Voog EG, Bryce AH, McDermott R, Ricci F, Rowe J, Zhang J, Piulats JM, Fizazi K, Merseburger AS, Higano CS, Krieger LE, Ryan CJ, Feng FY, Simmons AD, Loehr A, Despain D, Dowson M, Green F, Watkins SP, Golsorkhi T, Chowdhury S. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the phase II TRITON2 study. Clin Cancer Res. 2020;26(11):2487–96. https://doi.org/10.1158/1078-0432.CCR-20-0394.
Administration FAD. FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer: Food and Drug Administration; 2020 [updated 05/15/2020; cited 2021 09/12/2021]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate.
Jones P, Altamura S, Boueres J, Ferrigno F, Fonsi M, Giomini C, Lamartina S, Monteagudo E, Ontoria JM, Orsale MV, Palumbi MC, Pesci S, Roscilli G, Scarpelli R, Schultz-Fademrecht C, Toniatti C, Rowley M. Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and -2 mutant tumors. J Med Chem. 2009;52(22):7170–85. https://doi.org/10.1021/jm901188v.
Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, Hylands L, Riisnaes R, Forster M, Omlin A, Kreischer N, Thway K, Gevensleben H, Sun L, Loughney J, Chatterjee M, Toniatti C, Carpenter CL, Iannone R, Kaye SB, de Bono JS, Wenham RM. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–92. https://doi.org/10.1016/s1470-2045(13)70240-7.
Brenner JC, Ateeq B, Li Y, Yocum AK, Cao Q, Asangani IA, Patel S, Wang X, Liang H, Yu J, Palanisamy N, Siddiqui J, Yan W, Cao X, Mehra R, Sabolch A, Basrur V, Lonigro RJ, Yang J, Tomlins SA, Maher CA, Elenitoba-Johnson KS, Hussain M, Navone NM, Pienta KJ, Varambally S, Feng FY, Chinnaiyan AM. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19(5):664–78. https://doi.org/10.1016/j.ccr.2011.04.010.
Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, Chiuri VE, Jassem J, Flechon A, Redfern C, Goessl C, Burgents J, Kozarski R, Hodgson D, Learoyd M, Saad F. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19(7):975–86. https://doi.org/10.1016/S1470-2045(18)30365-6.
Kemp A. Lynparza in combination with abiraterone significantly delayed disease progression in all-comers in PROpel phase III trial in 1st-line metastatic castration-resistant prostate cancer: AstraZeneca; 2021 [updated 9/24/202112/28/2021]. Available from: https://www.astrazeneca.com/media-centre/press-releases/2021/lynparza-propel-trial-meets-primary-endpoint.html.
Saad F, Chi KN, Shore ND, Graff JN, Posadas EM, Lattouf JB, Espina BM, Zhu E, Yu A, Hazra A, De Meulder M, Mamidi R, Bradic B, Francis P, Hayreh V, Kalebasty Rezazadeh A. Niraparib with androgen receptor-axis-targeted therapy in patients with metastatic castration-resistant prostate cancer: safety and pharmacokinetic results from a phase 1b study (BEDIVERE). Cancer Chemother Pharmacol. 2021;88(1):25–37. https://doi.org/10.1007/s00280-021-04249-7.
Rao A, Morris D, Assikis VJ, Jha GG, Ryan CJ, Ablaza A-J, Habeck J, Loehr A, **ao J, Gangolli EA. Rucaparib plus enzalutamide in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): pharmacokinetics (PK) and safety data from the phase Ib RAMP study. J Clin Oncol. 2021;39(6_suppl):79. https://doi.org/10.1200/JCO.2021.39.6_suppl.79.
Petrylak DP, Loriot Y, Shaffer DR, Braiteh F, Powderly J, Harshman LC, Conkling P, Delord J-P, Gordon M, Kim JW, Sarkar I, Yuen K, Kadel EE, Mariathasan S, O’Hear C, Narayanan S, Fassò M, Carroll S, Powles T. Safety and clinical activity of atezolizumab in patients with metastatic castration-resistant prostate cancer: a phase I study. Clin Cancer Res. 2021;27(12):3360–9. https://doi.org/10.1158/1078-0432.Ccr-20-1981.
Ding L, Kim HJ, Wang Q, Kearns M, Jiang T, Ohlson CE, Li BB, **e S, Liu JF, Stover EH, Howitt BE, Bronson RT, Lazo S, Roberts TM, Freeman GJ, Konstantinopoulos PA, Matulonis UA, Zhao JJ. PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep. 2018;25(11):2972-80.e5. https://doi.org/10.1016/j.celrep.2018.11.054.
Karzai F, VanderWeele D, Madan RA, Owens H, Cordes LM, Hankin A, Couvillon A, Nichols E, Bilusic M, Beshiri ML, Kelly K, Krishnasamy V, Lee S, Lee MJ, Yuno A, Trepel JB, Merino MJ, Dittamore R, Marté J, Donahue RN, Schlom J, Killian KJ, Meltzer PS, Steinberg SM, Gulley JL, Lee JM, Dahut WL. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer. 2018;6(1):141. https://doi.org/10.1186/s40425-018-0463-2.
Yu EY, Piulats JM, Gravis G, Laguerre B, Arranz Arija JA, Oudard S, Fong PCC, Kolinsky MP, Augustin M, Feyerabend S, Kam AE, Gurney H, Tafreshi A, Retz M, Berry WR, Mar N, Wu H, Schloss C, Poehlein CH, De Bono JS. KEYNOTE-365 cohort A updated results: pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38(6_suppl):100. https://doi.org/10.1200/JCO.2020.38.6_suppl.100.
Yu EY, Park SH, Huang Y-H, Bennamoun M, Xu L, Kim J, Antonarakis ES. Phase III study of pembrolizumab (pembro) plus olaparib versus enzalutamide (enza) or abiraterone acetate (abi) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) who progressed on chemotherapy: KEYLYNK-010. J Clin Oncol. 2020;38(6_suppl):TPS256-TPS. https://doi.org/10.1200/JCO.2020.38.6_suppl.TPS256.
Petrylak DP, Perez-Gracia JL, Lacombe L, Bastos DA, Mahammedi H, Kwan EM, Zschäbitz S, Armstrong AJ, Pachynski RK, Goh JC, Burotto M, Gravis G, McCune SL, Vázquez Limón JC, Retz M, Saad F, Amin NP, Li J, Unsal-Kacmaz K, Fizazi K. 579MO CheckMate 9KD cohort A2 final analysis: nivolumab (NIVO) + rucaparib for chemotherapy (CT)-naïve metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2021;32:S629–30. https://doi.org/10.1016/j.annonc.2021.08.1092.
Shaya J, **e W, Saraiya B, Parikh M, Folefac E, Olson AC, Choudhury AD, Einstein DJ, Heath EI, Parikh RA, Kunos C, Ivy SP, LoRusso P, Kurzrock R, Shapiro G, McKay RR. A phase I/II study of combination olaparib and radium-223 in men with metastatic castration-resistant prostate cancer with bone metastases (COMRADE): a trial in progress. J Clin Oncol. 2021;39(6_suppl):TPS182-TPS. https://doi.org/10.1200/JCO.2021.39.6_suppl.TPS182.
Kelly WK, Leiby B, Einstein DJ, Szmulewitz RZ, Sartor AO, Yang ES-H, Sonpavde G. Radium-223 (Rad) and niraparib (Nira) treatment (tx) in castrate-resistant prostate cancer (CRPC) patients (pts) with and without prior chemotherapy (chemo). J Clin Oncol. 2020;38(15_suppl):5540. https://doi.org/10.1200/JCO.2020.38.15_suppl.5540.
Kim JW, McKay RR, Taplin M-E, Davis NB, Monk P, Appleman LJ, Lara P, Vaishampayan UN, Zhang J, Paul AK, Bubley G, Allen EMV, Huang Y, Zhang Z, Loda M, Shapiro G, LoRusso P, Ivy SP, Petrylak DP. Randomized phase II study of olaparib with or without cediranib in men with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38(6_suppl):111. https://doi.org/10.1200/JCO.2020.38.6_suppl.111.
McKay RR, Radke MR, Shyr Y, Zhao S, Taplin M-E, Davis NB, Monk P, Appleman LJ, Lara PLN, Vaishampayan UN, Zhang J, Paul AK, Bubley G, Huang Y, Shapiro G, LoRusso P, Ivy SP, Petrylak DP, Swisher EM, Kim JW. Biomarker analysis from a randomized phase II study of olaparib with or without cediranib in men with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2021;39(6_suppl):7. https://doi.org/10.1200/JCO.2021.39.6_suppl.7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Serhan Unlu declares that he has no conflict of interest.
Joseph W. Kim (JWK) is the study chair of one of the two NCI sponsored trials of olaparib, one of which is discussed in this review (NCI 9984).
JWK also has received consulting fees from the following companies: Voluntis, Sanofi, EMD Serono and Clovis Oncology.
JWK also received research funding from the following companies: Immune Design, Hummingbird, ADCT Therapeutics, Exelexis, Regeneron, Genetech/Roche, Cosmo Technologies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Genitourinary Cancers
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Unlu, S., Kim, J.W. Emerging Role of PARP Inhibitors in Metastatic Prostate Cancer. Curr Oncol Rep 24, 1619–1631 (2022). https://doi.org/10.1007/s11912-022-01305-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01305-0