Abstract
Purpose of Review
Prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy (TRT) is a promising investigational treatment for metastatic castration-resistant prostate cancer (mCRPC). This review describes the available data with PSMA TRT.
Recent Findings
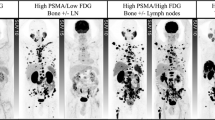
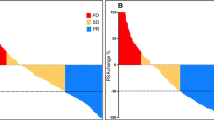
Conjugates used for PSMA TRT include antibodies or small molecules PSMA-radiolabeled with beta (most commonly 177Lu) or alpha emitters (commonly 225Ac). 177Lu-J591 demonstrated accurate targeting of known metastatic sites, based on post-treatment scintigraphy, in study populations that were not selected for PSMA expression, with evidence of dose-response and dose-limiting myelosuppression. Early phase studies of 177Lu-PSMA-617 have demonstrated favorable adverse event profiles and signs of clinical activity as evidenced by PSA responses and other short-term outcomes. A phase II randomized study of 177Lu-PSMA-617 showed a superior PSA50 response rate (66 vs 37%) over cabazitaxel in patients with docetaxel-pretreated, progressive mCRPC selected by PSMA and FDG PET/CT scans.
Summary
PSMA TRT is emerging as a promising investigational therapy for mCRPC. The first randomized data with 177Lu-PSMA-617 (phase 2) have been presented, and the first phase 3 trial has completed accrual with radiographic progression-free and overall survival as dual primary endpoints. Multiple additional phase 3 trials of PSMA-TRT are starting and studies investigating optimal patient selection and combination therapy continue.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sydes MR, Spears MR, Mason MD, Clarke NW, Dearnaley DP, de Bono JS, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29(5):1235–48.
Shaffer DR, Scher HI. Prostate cancer: a dynamic illness with shifting targets. Lancet Oncol. 2003;4(7):407–14.
Noonan K, North S, Bitting R, Armstrong A, Ellard S, Chi K. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24(7):1802–7.
Parker C, Nilsson S, Heinrich D, Helle SI, O'sullivan J, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–23.
De Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels J-P, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22.
Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373(18):1697–708.
Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7(5B):927–35.
O'Keefe DS, Bacich DJ, Huang SS, Heston WDW. A perspective on the evolving story of PSMA biology, PSMA-based imaging, and endoradiotherapeutic strategies. J Nucl Med. 2018;59(7):1007–13. https://doi.org/10.2967/jnumed.117.203877.
Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91(3):528–39.
Wright GL Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. In: urologic oncology: seminars and original investigations. Amsterdam: Elsevier; 1995. p. 18–28.
Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52(4):637–40.
Wright GL, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48(2):326–34. https://doi.org/10.1016/s0090-4295(96)00184-7.
Emmett L, Yin C, Crumbaker M, Hruby G, Kneebone A, Epstein R, et al. Rapid modulation of PSMA expression by androgen deprivation: serial 68Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. J Nucl Med. 2019;60(7):950–4.
Iravani A, Violet J, Azad A, Hofman MS. Lutetium-177 prostate-specific membrane antigen (PSMA) theranostics: Practical nuances and intricacies. Prostate Cancer Prostatic Dis. 2019:1–15.
Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58(10):1624–31.
Kahn D, Williams RD, Seldin DW, Libertino JA, Hirschhorn M, Dreicer R, et al. Radioimmunoscintigraphy with 111indium labeled CYT-356 for the detection of occult prostate cancer recurrence. J Urol. 1994;152(5):1490–5.
Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191(5):1446–53. https://doi.org/10.1016/j.juro.2013.10.065.
Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD. A phase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer Biother Radiopharm. 1999;14(2):99–111.
Deb N, Goris M, Trisler K, Fowler S, Saal J, Ning S, et al. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res. 1996;2(8):1289–97.
Liu H, Moy P, Kim S, **a Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57(17):3629–34.
Liu H, Rajasekaran AK, Moy P, **a Y, Kim S, Navarro V, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58(18):4055–60.
Vallabhajosula S, Kuji I, Hamacher KA, Konishi S, Kostakoglu L, Kothari PA, et al. Pharmacokinetics and biodistribution of 111In-and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J Nucl Med. 2005;46(4):634–41.
Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90—labeled anti—prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004;22(13):2522–31.
Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23(21):4591–601.
Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of lutetium-177–labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19(18):5182–91.
• Tagawa ST, Vallabhajosula S, Christos PJ, Jhanwar YS, Batra JS, Lam L, et al. Phase 1/2 study of fractionated dose lutetium-177–labeled anti–prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Cancer. 2019;125(15):2561–9 One of the largest prospective studies investigating 177Lu-J591 TRT. The dose-fractionation regimen allowed higher cumulative dose, as hypothesized, which resulted in improved efficacy (in terms of PSA decline and survival) with toxicity comparable to prior 177Lu-J591 studies.
Batra JS, Niaz MJ, Whang YE, Sheikh A, Thomas C, Christos P, et al. Phase I trial of docetaxel plus lutetium-177-labeled anti–prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Urol Oncol. 2020;38(11):848.e9–848.e16.
Niaz MJ, Batra JS, Walsh RD, Ramirez-Fort MK, Vallabhajosula S, Jhanwar YS, et al. Pilot study of hyperfractionated dosing of lutetium-177–labeled antiprostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Oncologist. 2020;25(6):477.
Roboz GJ, Bennett JM, Coleman M, Ritchie EK, Furman RR, Rossi A, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following initial treatment with chemotherapy plus radioimmunotherapy for indolent non-Hodgkin lymphoma. Leuk Res. 2007;31(8):1141–4.
Tagawa ST, Akhtar NH, Nikolopoulou A, Kaur G, Robinson B, Kahn R, et al. Bone marrow recovery and subsequent chemotherapy following radiolabeled anti-prostate-specific membrane antigen monoclonal antibody j591 in men with metastatic castration-resistant prostate cancer. Front Oncol. 2013;3:214.
• Tagawa ST, Osborne J, Fernandez E, Thomas C, Niaz MJ, Ciriaco A, et al. Phase I dose-escalation study of PSMA-targeted alpha emitter 225Ac-J591 in men with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38(Supplement 15):5560 The first prospective study investigating alpha-emitter therapy targeting PSMA, utilizing the J591 antibody. The highest planned dose, 93.3 KBq/kg, was tolerated without dose-limiting toxicity and there was preliminary evidence of efficacy in a PSMA-unselected population, most with prior exposure to PSMA-TRT.
Hammer S, Hagemann UB, Zitzmann-Kolbe S, Larsen A, Ellingsen C, Geraudie S, et al. Preclinical efficacy of a PSMA-targeted thorium-227 conjugate (PSMA-TTC), a targeted alpha therapy for prostate cancer. Clin Cancer Res. 2020;26(8):1985–96.
Kratochwil C, Haberkorn U, Giesel FL. Radionuclide therapy of metastatic prostate cancer. Semin Nucl Med. 2019;49:313–25.
Niaz MJ, Skafida M, Osborne J, Nanus D, Molina A, Thomas C, et al. PD16-11 comparison of prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy (TRT) with lutetium-177 (177 LU) via antibody J591 vs small molecule ligand PSMA-617. J Urol. 2020;203(Supplement 4):e367.
Pandit-Taskar N, O'Donoghue JA, Durack JC, Lyashchenko SK, Cheal SM, Beylergil V, et al. A phase I/II study for analytic validation of 89Zr-J591 immunoPET as a molecular imaging agent for metastatic prostate cancer. Clin Cancer Res. 2015;21(23):5277–85. https://doi.org/10.1158/1078-0432.CCR-15-0552.
Eder M, Schäfer M, Bauder-Wüst U, Hull W-E, Wängler C, Mier W, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23(4):688–97.
Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging. 2012;39(6):1085–6. https://doi.org/10.1007/s00259-012-2069-0.
Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5(6):856–63.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multi-centre study. Lancet. 2020;395:1208–16.
Rowe SP, Campbell SP, Mana-Ay M, Szabo Z, Allaf ME, Pienta KJ, et al. Prospective evaluation of PSMA-targeted 18F-DCFPyL PET/CT in men with biochemical failure after radical prostatectomy for prostate cancer. J Nucl Med. 2020;61(1):58–61.
Giesel FL, Knorr K, Spohn F, Will L, Maurer T, Flechsig P, et al. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med. 2019;60(3):362–8.
Eiber M, Krönke M, Wurzer A, Ulbrich L, Jooß L, Maurer T, et al. 18F-rhPSMA-7 positron emission tomography for the detection of biochemical recurrence of prostate cancer following radical prostatectomy. J Nucl Med. 2019;61(5):696–701.
Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, et al. Radiation dosimetry and first therapy results with a 124 I/131 I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41(7):1280–92.
Afshar-Oromieh A, Haberkorn U, Zechmann C, Armor T, Mier W, Spohn F, et al. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131 I-MIP-1095. Eur J Nucl Med Mol Imaging. 2017;44(6):950–9.
Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56(6):914–20.
Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57(8):1170–6.
Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with 177 Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5(1):36.
Ahmadzadehfar H, Eppard E, Kürpig S, Fimmers R, Yordanova A, Schlenkhoff CD, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7(11):12477–88.
Bräuer A, Grubert LS, Roll W, Schrader AJ, Schäfers M, Bögemann M, et al. 177 Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44(10):1663–70.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58(1):85–90.
Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45(1):12–9.
• Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19(6):825–33 The first prospective study of 177Lu-PSMA-617. In a population with heavily pre-treated mCRPC selected by PSMA and FDG PET, PSA response rate was high. The treatment was well-tolerated with onlh a minority with high-grade toxicity. Low grade xerostomia was much more common than that reported in retrospective studies.
• Violet J, Sandhu S, Iravani A, Ferdinandus J, Thang S-P, Kong G, et al. Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase II prospective trial of 177Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer. J Nucl Med. 2020;61(6):857–65 An expansion cohort of the original prospective 177Lu-PSMA-617 trial. Additional survival and outcome data were provided. Furthermore, outcomes for re-treatment therapy were defined.
Emmett L, Crumbaker M, Ho B, Willowson K, Eu P, Ratnayake L, et al. Results of a prospective phase 2 pilot trial of 177Lu–PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. 2019;17(1):15–22.
Halabi S, Lin C-Y, Kelly WK, Fizazi KS, Moul JW, Kaplan EB, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32(7):671–7.
Yadav MP, Ballal S, Bal C, Sahoo RK, Damle NA, Tripathi M, et al. Efficacy and safety of 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer patients. Clin Nucl Med. 2020;45(1):19–31.
Ferdinandus J, Eppard E, Gaertner FC, Kürpig S, Fimmers R, Yordanova A, et al. Predictors of response to radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617. J Nucl Med. 2017;58(2):312–9.
Kessel K, Seifert R, Schäfers M, Weckesser M, Schlack K, Boegemann M, et al. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617. Theranostics. 2019;9(17):4841–8.
Maffey-Steffan J, Scarpa L, Svirydenka A, Nilica B, Mair C, Buxbaum S, et al. The 68 Ga/177 Lu-theragnostic concept in PSMA-targeting of metastatic castration–resistant prostate cancer: impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur J Nucl Med Mol Imaging. 2020;47(3):695–712.
Rasul S, Hacker M, Kretschmer-Chott E, Leisser A, Grubmüller B, Kramer G, et al. Clinical outcome of standardized 177 Lu-PSMA-617 therapy in metastatic prostate cancer patients receiving 7400 MBq every 4 weeks. Eur J Nucl Med Mol Imaging. 2020;47(3):713–20.
Rathke H, Giesel FL, Flechsig P, Kopka K, Mier W, Hohenfellner M, et al. Repeated 177Lu-labeled PSMA-617 radioligand therapy using treatment activities of up to 9.3 GBq. J Nucl Med. 2018;59(3):459–65.
• Tagawa S, Osborne J, Hackett A, Niaz M, Cooley V, Christos P, et al. 849PD Preliminary results of a phase I/II dose-escalation study of fractionated dose 177Lu-PSMA-617 for progressive metastatic castration resistant prostate cancer (mCRPC). Ann Oncol. 2019;30(Supplement_5):mdz248. 006 The first study exploring dose-escalation of 177Lu-PSMA-617 therapy. Patients received a single fractionated-dose cycle of 22.2 GBq without dose limiting toxicity. In a PSMA-unselected population. 61% had over 50% PSA decline.
Violet J, Jackson P, Ferdinandus J, Sandhu S, Akhurst T, Iravani A, et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J Nucl Med. 2019;60(4):517–23.
Calais J, Fendler WP, Eiber M, Lassmann M, Dahlbom M, Esfandiari R, et al. RESIST-PC phase 2 trial: 177Lu-PSMA-617 radionuclide therapy for metastatic castrate-resistant prostate cancer. J Clin Oncol. 2019;37(Supplement 15):5028.
Hofman MS, Emmett L, Sandhu SK, Iravani A, Joshua AM, Goh JC, et al. TheraP: a randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: Initial results (ANZUP protocol 1603). J Clin Oncol. 2020;38(Supplement 15):5500.
Hofman MS, Emmett L, Violet J, Y. Zhang A, Lawrence NJ, Stockler M, et al. TheraP: a randomized phase 2 trial of 177Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 2019;124:5–13.
Rahbar K, Bodei L, Morris MJ. Is the vision of radioligand therapy for prostate cancer becoming a reality? An overview of the phase iii vision trial and its importance for the future of theranostics. J Nucl Med. 2019;60(11):1504–6.
Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57(12):1941–4.
Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59(5):795–802.
Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. 225 Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46(1):129–38.
Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga-and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56(8):1169–76.
Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of 177lu-psma radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med. 2019;60(7):955–62.
Heck MM, Retz M, D'Alessandria C, Rauscher I, Scheidhauer K, Maurer T, et al. Systemic radioligand therapy with 177Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J Urol. 2016;196(2):382–91.
Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57(7):1006–13.
• Heck MM, Tauber R, Schwaiger S, Retz M, D’Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2019;75(6):920–6 The largest study examining 177Lu-PSMA-I&T therapy, including 100 patients reported retrospectively. Therapeutic and toxicity profiles were defined.
Gafita A, Rauscher I, Retz M, Knorr K, Heck M, Wester H-J, et al. Early experience of rechallenge 177Lu-PSMA radioligand therapy after an initial good response in patients with advanced prostate cancer. J Nucl Med. 2019;60(5):644–8.
• Seifert R, Seitzer K, Herrmann K, Kessel K, Schäfers M, Kleesiek J, et al. Analysis of PSMA expression and outcome in patients with advanced Prostate Cancer receiving 177Lu-PSMA-617 Radioligand Therapy. Theranostics. 2020;10(17):7812 This study also examined the relationship between imaging features on PSMA-based PET scans and outcome after PSMA TRT. It introduced a new metric described as PSMAaverage, the average SUVmax of the five most avid lesions, and correlated that to survival.
Vlachostergios PJ, Niaz MJ, Skafida M, Mosallaie SA, Thomas C, Christos PJ, et al. Imaging expression of prostate-specific membrane antigen and response to PSMA-targeted beta-emitting radionuclide therapies in metastatic castration-resistant prostate cancer. In: The Prostate; 2021. In press. https://doi.org/10.1002/pros.24104.
Yordanova A, Linden P, Hauser S, Meisenheimer M, Kürpig S, Feldmann G, et al. Outcome and safety of rechallenge [177 Lu] Lu-PSMA-617 in patients with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46(5):1073–80.
Khreish F, Ebert N, Ries M, Maus S, Rosar F, Bohnenberger H, et al. 225 Ac-PSMA-617/177 Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. 2020;47(3):721–8.
Vlachostergios PJ, Conteduca V, Hackett AL, Manohar J, Lee A, Case A, et al. Prognostic value of BRCA2 and AR gene alterations in advanced prostate cancer patients treated with PSMA-targeted radionuclide therapies. Cancer Res. 2019;79(Supplement 13):4865.
Paschalis A, Sheehan B, Riisnaes R, Rodrigues DN, Gurel B, Bertan C, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019;76(4):469–78.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Michael Sun, Muhammad Junaid Niaz, and Muhammad Obaid Niaz declare no conflict of interest. Weill Cornell Medicine has received research funding for trials led by Scott T. Tagawa from Novartis/Advanced Accelerator Applications, Bayer, POINT Biopharma, Amgen, Atlab, Progenics, Pfizer, Clovis Oncology, Janssen, Seattle Genetics, Immunomedics, Ambrx, Inovio, Sanofi, Merck, Karyopharm, AbbVie, AstraZeneca, and Bristol-Myers Squibb; and he has received compensation for service as a consultant from Novartis/Advanced Accelerator Applications, Bayer, Blue Earth Diagnostics, POINT Biopharma, Amgen, Pfizer, QED, Clovis Oncology, Janssen, Seattle Genetics, Immunomedics, Ambrx, Sanofi, Karyopharm, and AbbVie.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Genitourinary Cancers
Rights and permissions
About this article
Cite this article
Sun, M., Niaz, M.J., Niaz, M.O. et al. Prostate-Specific Membrane Antigen (PSMA)-Targeted Radionuclide Therapies for Prostate Cancer. Curr Oncol Rep 23, 59 (2021). https://doi.org/10.1007/s11912-021-01042-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-021-01042-w