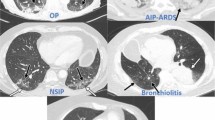
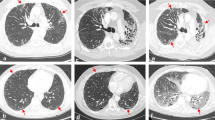
Abstract
Purpose of Review
Checkpoint inhibitor pneumonitis (CIP) is a toxicity of immune checkpoint blockade (ICB) that can be highly morbid and at times fatal. Here, we review the proposed biologic mechanisms of CIP, epidemiology and risk factors for CIP development, diagnostic work-up and management strategies for CIP, and future directions of CIP research.
Recent Findings
CIP incidence appears to be greater in real-world populations and may continue to rise as FDA approvals for ICB continue to expand to multiple malignancies. Multiple retrospective studies and case series have identified potential risk factors for CIP. Several society guidelines have helped to unify the classification of CIP severity and standardize treatment approaches but significant gaps remain, including formal validated diagnostic criteria for CIP.
Summary
While significant strides have been made in enhancing the knowledge and management of CIP, ongoing research is needed to continue to advance our understanding of the biologic underpinnings of CIP, as well as optimize diagnostic and management strategies for this potentially devastating toxicity.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with Pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–27.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–46.
• Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 Therapy. J Clin Oncol. 2017;35(7):709–17 Seminal retrospective study of ICB-treated NSCLC and melanoma patients that was one of the first publications to provide in-depth characterization of CIP incidence, clinical and radiographic features, pathologic assessment, and management outcomes.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39.
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35.
Khunger M, Jain P, Rakshit S, Pasupuleti V, Hernandez AV, Stevenson J, et al. Safety and efficacy of PD-1/PD-L1 inhibitors in treatment-naive and chemotherapy-refractory patients with non-small-cell lung cancer: a systematic review and meta-analysis. Clin Lung Cancer. 2018;19(3):e335–48.
• Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607–16 Comprehensive meta-analysis of ICB clinical trials outlining CIP incidence including severity and stratification by tumor type.
Zhang S, Liang F, Zhu J, Chen Q. Risk of pneumonitis associated with programmed cell death 1 inhibitors in cancer patients: a meta-analysis. Mol Cancer Ther. 2017;16(8):1588–95.
Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic impact and risk factors of immune-related pneumonitis in patients with non-small-cell lung cancer who received programmed death 1 inhibitors. Clin Lung Cancer. 2019;20(6):442–450.e4.
• Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930–9 Single-center analysis of ICB-treated NSCLC patients at Johns Hopkins that outlines potential CIP risk factors, incidence, clinical features, radiographic features, and management outcomes in a real-world population.
Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018;125:150–6.
Suresh K, Psoter KJ, Voong KR, Shankar B, Forde PM, Ettinger DS, et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol. 2019;14(3):494–502.
• Tone M, Izumo T, Awano N, Kuse N, Inomata M, Jo T, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer. 2019;10(10):2006–12 Single-center analysis of ICB-treated NSCLC patients that highlights potential CIP risk factors and showed that patients with high-grade CIP (≥ G3) had significantly decreased survival compared to those without high-grade CIP.
• Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–8 Comprehensive meta-analysis of World Health Organization database data that details spectrum, timing, and outcomes of fatal ICB-related toxicities.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8.
McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–9.
Bomze D, Hasan Ali O, Bate A, Flatz L. Association between immune-related adverse events during anti-PD-1 therapy and tumor mutational burden. JAMA Oncol. 2019. https://doi.org/10.1001/jamaoncol.2019.3221.
Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A. 2016;113(42):11919–24.
Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer. 2016;4:13,1 eCollection 2016.
Leroy V, Templier C, Faivre JB, Scherpereel A, Fournier C, Mortier L, et al. Pembrolizumab-induced pneumonitis. ERJ Open Res. 2017;3(2). https://doi.org/10.1183/23120541.00081,2016 eCollection 2017 Apr.
• Suresh K, Naidoo J, Zhong Q, **ong Y, Mammen J, de Flores MV, et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest. 2019;130:4305–15 Single-center study at Johns Hopkins that analyzed BAL fluid samples collected from patients with and without CIP. One of the first comparative analyses of the CIP immune landscape that lends insight into the underlying biologic mechanisms of CIP.
Tanaka K, Yanagihara T, Ikematsu Y, Inoue H, Ota K, Kashiwagi E, et al. Detection of identical T cell clones in peritumoral pleural effusion and pneumonitis lesions in a cancer patient during immune-checkpoint blockade. Oncotarget. 2018;9(55):30587–93.
Oda K, Kato K, Nakamura M, Jotatsu T, Noguchi S, Kawanami T, et al. Surface marker profiles on lung lymphocytes may predict the mechanism of immune-mediated pneumonitis triggered by tumor-infiltrating lymphocytes in lung cancer patients treated with pembrolizumab. Lung Cancer. 2018;118:171–2.
Laubli H, Koelzer VH, Matter MS, Herzig P, Dolder Schlienger B, Wiese MN, et al. The T cell repertoire in tumors overlaps with pulmonary inflammatory lesions in patients treated with checkpoint inhibitors. Oncoimmunology. 2017;7(2):e1386362.
Sukari A, Nagasaka M, Alhasan R, Patel D, Wozniak A, Ramchandren R, et al. Cancer site and adverse events induced by immune checkpoint inhibitors: a retrospective analysis of real-life experience at a single institution. Anticancer Res. 2019;39(2):781–90.
Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–85.
Ma K, Lu Y, Jiang S, Tang J, Li X, Zhang Y. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: a meta-analysis. Front Pharmacol. 2018;9:1430.
Abdel-Rahman O, Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ther Adv Respir Dis. 2016;10(3):183–93.
Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F, et al. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: a case-control study. Cancer Med. 2018;7(8):4115–20.
de Moel EC, Rozeman EA, Kapiteijn EH, Verdegaal EME, Grummels A, Bakker JA, et al. Autoantibody development under treatment with immune-checkpoint inhibitors. Cancer Immunol Res. 2019;7(1):6–11.
Ali OH, Bomze D, Ring S, Berner F, Fassler M, Diem S, et al. BP180-specific IgG is associated with skin adverse events, therapy response and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J Am Acad Dermatol. 2020;82(4):854-861. https://doi.org/10.1016/j.jaad.2019.08.045.
Tahir SA, Gao J, Miura Y, Blando J, Tidwell RSS, Zhao H, et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc Natl Acad Sci U S A. 2019;116(44):22246–51.
de Souza Costa VH, Baurakiades E, Viola Azevedo ML, Traiano G, Kowal Rosales J, Kunze Larsen KS, et al. Immunohistochemistry analysis of pulmonary infiltrates in necropsy samples of children with non-pandemic lethal respiratory infections (RSV; ADV; PIV1; PIV2; PIV3; FLU A; FLU B). J Clin Virol. 2014;61(2):211–5.
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32.
Su Q, Zhu EC, Wu JB, Li T, Hou YL, Wang DY, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front Immunol. 2019;10:108.
Pillai RN, Behera M, Owonikoko TK, Kamphorst AO, Pakkala S, Belani CP, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer. 2018;124(2):271–7.
**ao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014;211(5):943–59.
Toi Y, Sugawara S, Kawashima Y, Aiba T, Kawana S, Saito R, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist. 2018;23(11):1358–65.
Shafqat H, Gourdin T, Sion A. Immune-related adverse events are linked with improved progression-free survival in patients receiving anti-PD-1/PD-L1 therapy. Semin Oncol. 2018;45(3):156–63.
Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–4.
Rogado J, Sanchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Levi A, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019;109:21–7.
Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479–85.
Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung Cancer. Clin Lung Cancer. 2019;20(3):201–7.
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374–8.
Yamaguchi T, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Yatabe Y, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: a retrospective analysis. Lung Cancer. 2018;125:212–7.
Voong KR, Hazell SZ, Fu W, Hu C, Lin CT, Ding K, et al. Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2019;20(4):e470–9.
Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4(8):1112–5.
Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138–45.
Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845–51.
Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051–60.
Tonk EHJ, van Lindert ASR, JJC V, KPM S. Acute-onset pneumonitis while administering the first dose of durvalumab. Case Rep Oncol. 2019;12(2):621–4.
Reuss JE, Kunk PR, Stowman AM, Gru AA, Slingluff CL, Gaughan EM. Sarcoidosis in the setting of combination ipilimumab and nivolumab immunotherapy: a case report & review of the literature. J Immunother Cancer. 2016;4:94,9 eCollection 2016.
Naidoo J, Zhang J, Lipson EJ, Forde PM, Suresh K, Moseley KF, et al. A multidisciplinary toxicity team for cancer immunotherapy-related adverse events. J Natl Compr Cancer Netw. 2019;17(6):712–20.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–68.
Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119–42.
Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95 z.
National Comprehensive Cancer Network. Management of Immune Checkpoint Inhibitor-Related Toxicities (Version 2.2019) [Internet]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed 8 Dec 2019
Cadranel J, Canellas A, Matton L, Darrason M, Parrot A, Naccache JM, et al. Pulmonary complications of immune checkpoint inhibitors in patients with nonsmall cell lung cancer. Eur Respir Rev. 2019;28(153). https://doi.org/10.1183/16000617.0058-2019.
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103.
Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–44.
Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019. https://doi.org/10.1001/jamaoncol.2019.2785.
Johnson DB, Taylor KB, Cohen JV, Ayoubi N, Haugh AM, Wang DY, et al. Anti-PD-1-induced pneumonitis is associated with persistent imaging abnormalities in melanoma patients. Cancer Immunol Res. 2019;7(11):1755–9.
Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. 2019;25(3):551–7.
Ortega Sanchez G, Jahn K, Savic S, Zippelius A, Laubli H. Treatment of mycophenolate-resistant immune-related organizing pneumonia with infliximab. J Immunother Cancer. 2018;6(1):85–4.
Petri CR, Patell R, Batalini F, Rangachari D, Hallowell RW. Severe pulmonary toxicity from immune checkpoint inhibitor treated successfully with intravenous immunoglobulin: case report and review of the literature. Respir Med Case Rep. 2019;27:100834.
• Sears CR, Peikert T, Possick JD, Naidoo J, Nishino M, Patel SP, et al. Knowledge gaps and research priorities in immune checkpoint inhibitor-related pneumonitis. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2019;200(6):e31–43 Consensus statement published by the American Thoracic Society that summarizes important findings in CIP research, research gaps, and key research questions to stimulate further investigation.
Hsiehchen D, Watters MK, Lu R, **e Y, Gerber DE. Variation in the assessment of immune-related adverse event occurrence, grade, and timing in patients receiving immune checkpoint inhibitors. JAMA Netw Open. 2019;2(9):e1911519.
Liu J, Blake SJ, Smyth MJ, Teng MW. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin Transl Immunology. 2014;3(8):e22.
Wang F, Luo Y, Tian X, Ma S, Sun Y, You C, et al. Impact of radiotherapy concurrent with anti-PD-1 therapy on the lung tissue of tumor-bearing mice. Radiat Res. 2019;191(3):271–7.
Weaver JL, Zadrozny LM, Gabrielson K, Semple KM, Shea KI, Howard KE. BLT-immune humanized mice as a model for Nivolumab-induced immune-mediated adverse events: comparison of the NOG and NOG-EXL strains. Toxicol Sci. 2019;169(1):194–208.
Schoenfeld JD, Nishino M, Severgnini M, Manos M, Mak RH, Hodi FS. Pneumonitis resulting from radiation and immune checkpoint blockade illustrates characteristic clinical, radiologic and circulating biomarker features. J Immunother Cancer. 2019;7(1):112–3.
Oyanagi J, Koh Y, Sato K, Mori K, Teraoka S, Akamatsu H, et al. Predictive value of serum protein levels in patients with advanced non-small cell lung cancer treated with nivolumab. Lung Cancer. 2019;132:107–13.
Lauritsen J, Kier MG, Bandak M, Mortensen MS, Thomsen FB, Mortensen J, et al. Pulmonary function in patients with germ cell cancer treated with bleomycin, etoposide, and cisplatin. J Clin Oncol. 2016;34(13):1492–9.
Franzen D, Schad K, Kowalski B, Clarenbach CF, Stupp R, Dummer R, et al. Ipilimumab and early signs of pulmonary toxicity in patients with metastastic melanoma: a prospective observational study. Cancer Immunol Immunother. 2018;67(1):127–34.
Reuss J, Suresh K, Psoter K, Forde P, Naidoo J. P1.16-06 early changes in pulmonary function are associated with development of pneumonitis in NSCLC patients receiving immune checkpoint blockade. J Thorac Oncol. 2019;14(10):S587–8.
Colen RR, Fujii T, Bilen MA, Kotrotsou A, Abrol S, Hess KR, et al. Radiomics to predict immunotherapy-induced pneumonitis: proof of concept. Investig New Drugs. 2018;36(4):601–7.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Joshua E. Reuss declares that he has no conflict of interest. Karthik Suresh declares that he has no conflict of interest. Jarushka Naidoo has received research funding from Merck and AstraZeneca; has received compensation from Bristol-Myers Squibb, AstraZeneca, and Roche/Genentech for consulting/advisory board work; and has received honoraria from Bristol-Myers Squibb and AstraZeneca.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lung Cancer
Rights and permissions
About this article
Cite this article
Reuss, J.E., Suresh, K. & Naidoo, J. Checkpoint Inhibitor Pneumonitis: Mechanisms, Characteristics, Management Strategies, and Beyond. Curr Oncol Rep 22, 56 (2020). https://doi.org/10.1007/s11912-020-00920-z
Published:
DOI: https://doi.org/10.1007/s11912-020-00920-z