Abstract
Purpose of Review
Hypertension is one of the most challenging health problems inducing cerebrovascular disease and high percentage of death when associated with diabetes, dyslipidemias, and obesity. Orexin/hypocretin is a peptide expressed by a small number of neurons of the dorsolateral hypothalamus, a brain feeding and autonomic “fight-or-flight” regulatory center. According to this function, orexin has been demonstrated to evoke cardiovascular responses, heart rate, hypertension, hyperarousal, hyperphagia, and obesity. The focus of this review is to provide an overview about the mechanism through which orexin regulates food intake and cardiovascular responses and its role in the pathogenesis of obesity and hypertension which could be of great interest to establish possible new therapies.
Recent Findings
In normal rats and mice, central administration of orexin increases food intake, blood pressure, and sympathetic nerve activity and these effects are blocked by selective orexin receptor antagonist SB-334867 or almorexant. Moreover, upregulation of orexin signaling, in combination with elevation of epinephrine and norepinephrine circulating levels, occurs in rats exposed to chronic stress, in models of spontaneous hypertension (SHR and BPH/2J Schlager mice) and in obese mice (ob/ob or mice fed with high fat diet). Therefore, hyperactivity of orexinergic neurons could be a factor in the development of obesity and essential hypertension.
Summary
Because of their widespread projections to the brain regions involved in appetite and cardiovascular responses, as far down as sympathetic preganglionic neurons in the spinal cord, orexin evokes sympathetically mediated cardiovascular responses. Lasting upregulation of orexin signaling can lead to hyperphagia, obesity, and hypertensive state. Dual orexin receptor antagonists (DORAs) and selective orexin receptor antagonists (SORAs) have antihypertensive effects that could be of clinical use for regulation of food intake and hypertension, supporting the role of orexinergic neurons as critical checkpoint in the neurogenic control of metabolic and cardiovascular functions.

Similar content being viewed by others
Abbreviations
- 2-AG:
-
2-arachidonoylglycerol
- ACE:
-
Angiotensin-converting enzyme
- ACTH:
-
Adreno corticotropic hormone
- Amb:
-
Nucleus ambiguous
- Ang-II:
-
Angiotensin II
- ARC:
-
Arcuate nucleus
- AT1:
-
Angiotensin type 1 receptor
- AVP:
-
Arginine-vasopressin
- BPH/2 J:
-
High blood pressure mouse
- BPN/3 J:
-
Normotensive blood pressure mouse
- CRF:
-
Corticotropin-releasing factor
- CRH:
-
Adrenocorticotropin-releasing hormone
- DMN:
-
Dorsomedial nucleus
- Epi:
-
Epinephrine
- GFP:
-
Green fluorescent protein
- Hcrt1:
-
Hypocretin 1
- Hcrt2:
-
Hypocretin 2
- HFD:
-
High-fat diet
- HPA:
-
Hypothalamic-pituitary-adrenal axis
- HR:
-
Heart rate
- i.c.v.:
-
Intracerebroventricular
- IML:
-
Intermediolateral column
- i.p.:
-
Intraperitoneal
- i.v.:
-
Intravenous
- LC:
-
Locus coeruleus
- LHA:
-
Lateral hypothalamic area
- LZRs:
-
Lean Zucker rats
- MAP:
-
Mean arterial pressure
- MSH:
-
Melanocyte Stimulating Hormone
- NE:
-
Norepinephrine
- NPY:
-
Neuropeptide-Y
- NTS:
-
Nucleus tractus solitaries
- Ox1R:
-
Orexin-1 receptor
- Ox2R:
-
Orexin-2 receptor
- OXRs:
-
Ox1R and Ox2R
- OX-A:
-
Orexin-A
- OX-B:
-
Orexin-B
- OXs:
-
OX-A and OX-B
- OZRs:
-
Obese Zucker rats
- PAG:
-
Periaqueductal gray
- POMC:
-
Proopiomelanocortin
- PVN:
-
Paraventricular nucleus
- RAS:
-
Renin-angiotensin system
- RSNA:
-
Response sympathetic nervous autonomous
- RVLM:
-
Rostral ventrolateral medulla
- RVMM:
-
Rostral ventromedial medulla
- s.c.:
-
Subcutaneous
- SCN:
-
Supra-chiasmatic nucleus
- SFD:
-
Standard-fat diet
- SHR:
-
Spontaneously hypertensive rat
- SIHR:
-
Stress-induced hypertensive rats
- SNA:
-
Sympathetic nerve activity
- SPNs:
-
Sympathetic preganglionic neurons
- VMN:
-
Paraventricular nucleus
- ZF:
-
Zona fasciculate
- ZR:
-
Zona reticularis
- WKY:
-
Wistar Kyoto rats
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett 2nd FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–7.
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–85.
Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F, Elshourbagy NA, Ellis CE, Middlemiss DN, Brown F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol. 1999;128(1):1–3.
Smart D, Jerman JC, Brough SJ, Neville WA, Jewitt F, Porter RA. The hypocretins are weak agonists at recombinant human orexin-1 and orexin-2 receptors. Br J Pharmacol. 2000;129(7):1289–91.
Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438(1–2):71–5.
Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav. 2000;37(4):335–44.
Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25.
Sunter D, Morgan I, Edwards CM, Dakin CL, Murphy KG, Gardiner J, Taheri S, Rayes E, Bloom SR. Orexins: effects on behavior and localisation of orexin receptor 2 messenger ribonucleic acid in the rat brainstem. Brain Res. 2001;907(1–2):27–34.
van den Top M, Nolan MF, Lee K, Richardson PJ, Buijs RM, Davies CH, Spanswick D. Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol. 2003;549(Pt 3):809–21.
Ch'ng SS, Lawrence AJ. Distribution of the orexin-1 receptor (OX1R) in the mouse forebrain and rostral brainstem: a characterisation of OX1R-eGFP mice. J Chem Neuroanat. 2015;66–67:1–9.
Darwinkel A, Stanić D, Booth LC, May CN, Lawrence AJ, Yao ST. Distribution of orexin-1 receptor-green fluorescent protein- (OX1-GFP) expressing neurons in the mouse brain stem and pons: Co-localization with tyrosine hydroxylase and neuronal nitric oxide synthase. Neuroscience. 2014;278:253–64.
Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res Mol Brain Res. 2001;88(1–2):176–82.
Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103(3):777–97.
Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104(1–3):131–44.
Bäckberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15(2):315–28.
Beig MI, Dampney BW, Carrive P. Both Ox1r and Ox2r orexin receptors contribute to the cardiovascular and locomotor components of the novelty stress response in the rat. Neuropharmacology. 2015;89:146–56.
Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res. 2003;991(1–2):84–95.
Shahid IZ, Rahman AA, Pilowsky PM. Intrathecal orexin a increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato-sympathetic reflex. Br J Pharmacol. 2011;162(4):961–73.
• Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther. 2010;334(2):522–9. One of the earliest studies showing the role of orexins in the control of blood pressor and cardiovascular system by promoting the sympathetic outflow through a direct depolarization of adrenergic and noradrenergic RVLM neurons.
**ao F, Jiang M, Du D, **a C, Wang J, Cao Y, Shen L, Zhu D. Orexin A regulates cardiovascular responses in stress-induced hypertensive rats. Neuropharmacology. 2013;67:16–24.
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015.
Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK. Orexin A-like immunoreactivity in the rat brain. Neurosci Lett. 1999;260(3):161–4.
Llewellyn-Smith IJ, Martin CL, Marcus JN, Yanagisawa M, Minson JB, Scammell TE. Orexin-immunoreactive inputs to rat sympathetic preganglionic neurons. Neurosci Lett. 2003;351(2):115–9.
• van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19(8):3171–82. The study reports the first evidence of direct projections form orexin neurons to the spinal intermediolateral cells of the thoracic laminae. This pattern of innervation is very similar between mouse, rat, and human and provides the anatomical support for orexin in the regulation of sympathetic function.
Date Y, Mondal MS, Matsukura S, Nakazato M. Distribution of orexin-A and orexin-B (hypocretins) in the rat spinal cord. Neurosci Lett. 2000;288(2):87–90.
de Lecea L. A decade of hypocretins: past, present and future of the neurobiology of arousal. Acta Physiol (Oxf). 2010;198(3):203–8.
Sakurai T, Mieda M, Tsu**o N. The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–61.
de Lecea L. Hypocretins and the neurobiology of sleep-wake mechanisms. Prog Brain Res. 2012;198:15–24.
Tsu**o N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61(2):162–76.
Teske JA, Billington CJ, Kotz CM. Hypocretin/orexin and energy expenditure. Acta Physiol (Oxf). 2010;198(3):303–12.
Girault EM, Yi CX, Fliers E, Kalsbeek A. Orexins, feeding, and energy balance. Prog Brain Res. 2012;198:47–64.
• Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96(2):748–53. This study describes the widespread network of orexin projections to the different regions of the brain involved in the control of autonomic and neuroendocrine functions.
Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39(2):275–80.
Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49(4):916–25.
Matsumura K, Tsuchihashi T, Abe I. Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension. 2001;37(6):1382–7.
•• Shirasaka T, Kunitake T, Takasaki M, Kannan H. Neuronal effects of orexins: relevant to sympathetic and cardiovascular functions. Regul Pept. 2002;104(1–3):91–5. This review highlights the essential role of orexin in the regulation of cardiovascular responses and sympathetic functions, underlying its role in arterial pressure, heart rate, and control of epinephrine and norepinephrine production.
Shahid IZ, Rahman AA, Pilowsky PM. Orexin and central regulation of cardiorespiratory system. Vitam Horm. 2012a;89:159–84.
Shirasaka T, Takasaki M, Kannan H. Cardiovascular effects of leptin and orexins. Am J Physiol Regul Integr Comp Physiol. 2003;284(3):R639–51.
Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96(19):10911–6.
Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415(2):145–59.
Tsu**o N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:28.
• Carrive P. Orexin, orexin receptor antagonists and central cardiovascular contro. Front Neurosci. 2013;7:257. The antihypertensive therapeutical utilization of the dual orexin receptor antagonists (DORAs) and of the selective orexin receptor antagonists (SORAs) is suggested for the first time in this study.
van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in develo** mouse GFP-expressing locus coeruleus. J Physiol. 2002;541(Pt 1):169–85.
Chen XW, Huang W, Yan JA, Fan HX, Guo N, Lü J, **u Y, Gu JL, Zhang CX, Ruan HZ, Hu ZA, Yu ZP, Zhou Z. Reinvestigation of the effect of orexin A on catecholamine release from adrenal chromaffin cells. Neurosci Lett. 2008;436(2):181–4.
• Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci. 1984;4:474–94. One of the earliest studies showing that RVLM C1 adrenergic neurons exert an excitatory influence on the sympathetic vasomotor fibers and adrenal medulla.
• Puskás N, Papp RS, Gallatz K, Palkovits M. Interactions between orexin-immunoreactive fibers and adrenaline or noradrenaline-expressing neurons of the lower brainstem in rats and mice. Peptides. 2010;31(8):1589–97. The study revelas an interaction between orexin containing fibers and EPI/NE neurons in the main brain areas involved in the central cardiovascular regulation.
Bochorishvili G, Nguyen T, Coates MB, Viar KE, Stornetta RL, Guyenet PG. The orexinergic neurons receive synaptic input from C1 cells in rats. J Comp Neurol. 2014;522(17):3834–46.
Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–46.
Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension. 2011;57(6):1026–33.
Fisher JP, Paton JF. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens. 2012;26(8):463–75.
Matsumura K, Tsuchihashi T, Fujii K, Iida M. Neural regulation of blood pressure by leptin and the related peptides. Regul Pept. 2003;114(2–3):79–86.
Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol. 2002;545(Pt 3):855–67.
Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area: I. Descending projections J Comp Neurol. 1992;315(3):313–32.
Guyenet PG, Darnall RA, Riley TA. Rostral ventrolateral medulla and sympathorespiratory integration in rats. Am J Phys. 1990;259(5 Pt 2):R1063–74.
Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74(2):323–64.
•• Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Phys. 1999;277(6 Pt 2):R1780–5. One of the earliest studies showing the effects of central orexins on blood pressure and sympathetic nerve activity in conscious rats. Central injection of orexin-A induces cardiovascular responses, regulates renal sympathetic nerve activity, and increases plasma catecholamine release in conscious rats.
Shahid IZ, Rahman AA, Pilowsky PM. Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br. J. Pharmacol. 2012b;165:2292–303.
Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept. 2002;104(1–3):75–81.
Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2000;278(3):R692–7.
Lin Y, Matsumura K, Tsuchihashi T, Abe I, Iida M. Chronic central infusion of orexin-a increases arterial pressure in rats. Brain Res Bull. 2002;57(5):619–22.
Lee YH, Tsai MC, Li TL, Dai YW, Huang SC, Hwang LL. Spontaneously hypertensive rats have more orexin neurons in the hypothalamus and enhanced orexinergic input and orexin 2 receptor-associated nitric oxide signalling in the rostral ventrolateral medulla. Exp Physiol. 2015;100(9):993–1007.
Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285(3):R581–93.
Zhang W, Shimoyama M, Fukuda Y, Kuwaki T. Multiple components of the defense response depend on orexin: evidence from orexin knockout mice and orexin neuron-ablated mice. Auton Neurosci. 2006;126-127:139–45.
Hirota K, Kushikata T, Kudo M, Kudo T, Smart D, et al. Effects of central hypocretin-1 administration on hemodynamic responses in young-adult and middle-aged rats. Brain Res. 2003;981:143–50.
•• Li A, Hindmarch CC, Nattie EE, Paton JF. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol. 2013;591:4237–48. doi:10.1113/jphysiol.2013.256271. Study provides the role of orexin system in the genesis and maintenance of the high blood pressure in the SHR. The key findings of the study are the strong increase of orexin mRNA expression in the RVLM of hypertensive rats and the possibility to reduce blood pressure and levels of adrenaline and noradrenaline in the CSF and plasma through antagonism of orexin receptors.
Lee YH, Dai YW, Huang SC, Li TL, Hwang LL. Blockade of central orexin 2 receptors reduces arterial pressure in spontaneously hypertensive rats. Exp Physiol. 2013;98:1145–55.
Marques FZ, Campain AE, Davern PJ, Yang YH, Head GA, Morris BJ. Genes influencing circadian differences in blood pressure in hypertensive mice. PLoS One. 2011a;6(4):e19203.
Marques FZ, Campain AE, Davern PJ, Yang YH, Head GA, Morris BJ. Global identification of the genes and pathways differentially expressed in hypothalamus in early and established neurogenic hypertension. Physiol Genomics. 2011b;43(12):766–71.
Yamamoto H, Okuzaki D, Yamanishi K, Xu Y, Watanabe Y, Yoshida M, Yamashita A, Goto N, Nishiguchi S, Shimada K, Nojima H, Yasunaga T, Okamura H, Matsunaga H, Yamanishi H. Genetic analysis of genes causing hypertension and stroke in spontaneously hypertensive rats. Int J Mol Med. 2013;31(5):1057–65.
Zimmerman RS, Frohlich ED. Stress and hypertension. J Hypertens Suppl. 1990;8(4):S103–7.
Boone JL. Stress and hypertension. Prim Care. 1991;18(3):623–49.
Markovitz JH, Matthews KA, Kannel WB, Cobb JL, D'Agostino RB. Psychological predictors of hypertension in the Framingham study. Is there tension in hypertension? JAMA. 1993;270(20):2439–43.
Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25(3–4):132–49.
• Al-Barazanji KA, Wilson S, Baker J, Jessop DS, Harbuz MS. Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. J Neuroendocrinol. 2001;13(5):421–4. The study describes the important role of orexin-A in the activation of the hypothalamic-pituitary-adrenal axis and enhancement of ACTH, CRF, and AVP levels underlying the hypertensive state.
Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept. 2002;104(1–3):97–103.
Russell SH, Small CJ, Kennedy AR, Stanley SA, Seth A, Murphy KG, Taheri S, Ghatei MA, Bloom SR. Orexin A interactions in the hypothalamo-pituitary gonadal axis. Endocrinology. 2001;142(12):5294–302.
Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R382–7.
Kok SW, Roelfsema F, Overeem S, Lammers GJ, Strijers RL, Frölich M, Meinders AE, Pijl H. Dynamics of the pituitary-adrenal ensemble in hypocretin-deficient narcoleptic humans: blunted basal adrenocorticotropin release and evidence for normal time-kee** by the master pacemaker. J Clin Endocrinol Metab. 2002;87(11):5085–91.
Malendowicz LK, Tortorella C, Nussdorfer GG. Orexins stimulate corticosterone secretion of rat adrenocortical cells, through the activation of the adenylate cyclase-dependent signaling cascade. J Steroid Biochem Mol Biol. 1999a;70(4–6):185–8.
Nowak M, Hochól A, Tortorella C, Jedrzejczak N, Ziolkowska A, Nussdorfer GG, Malendowicz LK. Modulatory effects of orexins on the function of rat pituitary-adrenocortical axis under basal and stressful conditions. Biomed Res. 2000;21:89–93.
• Malendowicz LK, Hochol A, Ziolkowska A, Nowak M, Gottardo L, Nussdorfer GG. Prolonged orexin administration stimulates steroid-hormone secretion, acting directly on the rat adrenal gland. Int J Mol Med. 2001;7(4):401–4. The study shows how systemic orexin injection increases plasma concentration of both aldosterone and corticosterone act on the adrenocortical cells.
Nussdorfer GG. Paracrine control of adrenal cortical function by medullary chromaffin cells. Pharmacol Rev. 1996;48:495–530.
Crowley SD, Coffman TM. Recent advances involving the renin–angiotensin system. Exp Cell Res. 2012;318(9):1049–56.
Shanmugam S, Sandberg K. Ontogeny of angiotensin II receptors. Cell Biol Int. 1996;20:169–76.
Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Front Neuroendocrinol. 1997;18:383–439.
DiBona GF. Nervous kidney. Interaction between renal sympathetic nerves and the renin-angiotensin system in the control of renal function. Hypertension. 2000;36(6):1083–8.
Jensen LL, Harding JW, Wright JW. Central effects of a specific angiotensin receptor antagonist, sarthran (Sar1, Thr8AII) in normotensive and spontaneously hypertensive rat strains. Brain Res. 1988;448(2):359–63.
Yoshida T, Semprun-Prieto L, Wainford RD, Sukhanov S, Kapusta DR, Delafontaine P. Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus. Endocrinology. 2012;153(3):1411–20.
Tanida M, Niijima A, Shen J, Yamada S, Sawai H, Fukuda Y, Nagai K. Dose-different effects of orexin-A on the renal sympathetic nerve and blood pressure in urethane-anesthetized rats. Exp Biol Med (Maywood). 2006;231(10):1616–25.
Jackson KL, Dampney BW, Moretti JL, Stevenson ER, Davern PJ, Carrive P, Head GA. Contribution of orexin to the neurogenic hypertension in BPH/2J mice. Hypertension. 2016;67(5):959–69.
Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14(7):1075–81.
Rettig R, Gerstberger R, Meyer JU, Intaglietta M, Printz MP. Central effects of angiotensin II in conscious hamsters: drinking, pressor response, and release of vasopressin. J Comp Physiol B. 1989;158:703–9.
Jensen LL, Harding JW, Wright JW. Role of paraventricular nucleus in control of blood pressure and drinking in rats. Am J Phys. 1992;262(6 Pt 2):F1068–75.
Malendowicz LK, Tortorella C, Nussdorfer GG. Orexins stimulate corticosterone secretion of rat adrenocortical cells, through the activation of the adenylate cyclase-dependent signaling cascade. J Steroid Biochem Mol Biol. 1999b;70:185–8.
Mazzocchi G, Malendowicz LK, Gottardo L, Aragona F, Nussdorfer GG. Orexin A stimulates cortisol secretion from human adrenocortical cells through activation of the adenylate cyclase-dependent signaling cascade. J Clin Endocrinol Metab. 2001;86:778–82.
Lopez M, Señaris R, Gallego R, Garcia-Caballero T, Lago F, Seoane L, Casanueva F, Dieguez C. Orexin receptors are expressed in the adrenal medulla of the rat. Endocrinology. 1999;140:5991–4.
Karteris E, Machado RJ, Chen J, Zervou S, Hillhouse EW, Randeva HS. Food deprivation differentially modulates orexin receptor expression and signaling in rat hypothalamus and adrenal cortex. Am J Phys. 2005;288:E1089–100.
Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors and sequelae. Endocrinology. 1999;135:4015–23.
Spinazzi R, Rucinski M, Neri G, Malendowicz LK, Nussdorfer GG. Prepro-orexin and orexin receptors are expressed in cortisol-secreting adrenocortical adenomas and orexins stimulate in vitro cortisol secretion and growth of tumor cells. J Clin Endocrinol Metab. 2005;90:3544–9.
Morello G, Imperatore R, Palomba L, Finelli C, Labruna G, Pasanisi F, Sacchetti L, Buono L, Piscitelli F, Orlando P, Di Marzo V, Cristino L. Orexin-A represses satiety-inducing POMC neurons and contributes to obesity via stimulation of endocannabinoid signaling. Proc Natl Acad Sci U S A. 2016;113(17):4759–64.
• Cristino L, Busetto G, Imperatore R, Ferrandino I, Palomba L, Silvestri C, Petrosino S, Orlando P, Bentivoglio M, Mackie K, Di Marzo V. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc Natl Acad Sci U S A. 2013;110(24):E2229–38. This study reports the elevation of circulating Epi and NE plasma levels in obese mice ( ob/ob and HFD) and its lowering to normal concentrations by intraperitoneal injection of SB-334867 (OX-1R antagonist).
Nanmoku T, Isobe K, Sakurai T, Yamanaka A, Takekoshi K, Kawakami Y, Ishii K, Goto K, Nakai T Orexins suppress catecholamine synthesis and secretion in cultured PC12 cells. Biochem Biophys Res Commun 2000;274: 310–315.
Kawada Y, Ueno S, Asayama K, Tsutsui M, Utsunomiya K, Toyohira Y, Morisada N, Tanaka K, Shirahata A, Yanagihara N. Stimulation of catecholamine synthesis by orexin-A in bovine adrenal medullary cells through orexin receptor 1. Biochem Pharmacol. 2003;66:141–7.
Bastianini S, Silvani A, Berteotti C, Elghozi JL, Franzini C, Lenzi P, Lo Martire V, Zoccoli G. Sleep related changes in blood pressure in hypocretin-deficient narcoleptic mice. Sleep. 2011;34:213–8.
Silvani A, Bastianini S, Berteotti C, Cenacchi G, Leone O, Lo Martire V, Papa V, Zoccoli G. Sleep and cardiovascular phenotype in middle-aged hypocretin-deficient narcoleptic mice. J Sleep Res. 2013;1:98–106.
Grimaldi D, Pierangeli G, Barletta G, Terlizzi R, Plazzi G, Cevoli S, Franceschini C, Montagna P, Cortelli P. Spectral analysis of heart rate variability reveals an enhanced sympathetic activity in narcolepsy with cataplexy. Clin Neurophysiol. 2010;121:1142–7.
Grimaldi D, Calandra-Buonaura G, Provini F, Agati P, Pierangeli G, Franceschini C, Barletta G, Plazzi G, Montagna P, Cortelli P. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: effects of hypocretin deficiency in humans. Sleep. 2012;35:519–28. doi:10.5665/sleep.1738.
Dauvilliers Y, Jaussent I, Krams B, Scholz S, Lado S, Levy P, Pepin JL. Non-dip** blood pressure profile in narcolepsy with cataplexy. PLoS One. 2012;7:e38977.
Lo Martire V, Silvani A, Bastianini S, Berteotti C, Zoccoli G. Effects of ambient temperature on sleep and cardiovascular regulation in mice: the role of hypocretin/orexin neurons. PLoS One. 2012;7:e47032.
Sorensen GL, Knudsen S, Petersen ER, Kempfner J, Gammeltoft S, Sorensen HB, Jennum P. Attenuated heart rate response is associated with hypocretin deficiency in patients with narcolepsy. Sleep. 2013;36:91–8.
Tucci V, Stegagno L, Vandi S, Ferrillo F, Palomba D, Vignatelli L, Ferini-Strambi L, Montagna P, Plazzi G. Emotional information processing in patients with narcolepsy: a psychophysiologic investigation. Sleep. 2003;26:558–64.
Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43.
McIntyre AM. Burden of illness review of obesity: are the true costs realised? J R Soc Health. 1998;118(2):76–84.
Macdonald IA. Obesity: are we any closer to identifying causes and effective treatments? Trends Pharmacol Sci. 2000;21(9):334–6.
Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension. 2000;35(1 Pt 2):403–8.
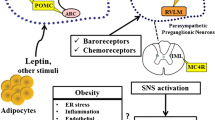
Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45(1):9–14.
Morgan DA, Anderson EA, Mark AL. Renal sympathetic nerve activity is increased in obese Zucker rats. Hypertension. 1995;25(4 Pt 2):834–8.
Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension. 1996;28(6):1047–54.
Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2007;293(4):H2543–9.
Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25(4 Pt 2):893–7.
Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55(4):862–8.
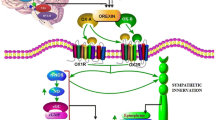
•• Zhou JJ, Yuan F, Zhang Y, Li DP. Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology. 2015;99:481–90. The study shows, for the first time, that upregulation of OX-1R in the PVN enhances the sympathetic outflow during obesity. The study suggests that OX-1R may represent a new target to treat obesity-related hypertension.
Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res. 1998;120(2):164–72.
Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100(3):549–56.
Beck B, Richy S, Dimitrov T, Stricker-Krongrad A. Opposite regulation of hypothalamic orexin and neuropeptide Y receptors and peptide expressions in obese Zucker rats. Biochem Biophys Res Commun. 2001;286(3):518–23.
WHO (world Health Organization) (1998) Obesity—preventing and managing the global epidemic. Report of a WHO consultation on obesity. 1997.
Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20(1):68–100.
Beck B. Neuropeptides and obesity. Nutrition. 2000;16(10):916–23.
Mercer JG, Speakman JR. Hypothalamic neuropeptide mechanisms for regulating energy balance: from rodent models to human obesity. Neurosci Biobehav Rev. 2001;25(2):101–16.
Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54.
Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96(1–2):45–51.
Shiraishi T, Oomura Y, Sasaki K, Wayner MJ. Effects of leptin and orexin-A on food intake and feeding related hypothalamic neurons. Physiol Behav. 2000;71(3–4):251–61.
Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsu**o N, Mieda M, Tominaga M, Ki Y, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–13.
Huang H, Acuna-Goycolea C, Li Y, Cheng HM, Obrietan K, van den Pol AN. Cannabinoids excite hypothalamic melanin-concentrating hormone but inhibit hypocretin/orexin neurons: implications for cannabinoid actions on food intake and cognitive arousal. J Neurosci. 2007;27(18):4870–81.
Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–71.
Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–8.
Iigaya K, Horiuchi J, McDowall LM, Lam ACB, Sediqi Y, Polson JW, Carrive P, Dampney RA. Blockade of orexin receptors with almorexant reduces cardiorespiratory responses evoked from the hypothalamus but not baro- or chemoreceptor reflex responses. Am J Phys. 2012;303:R1011–22.
Nisimaru N, Mittal C, Shirai Y, Sooksawate T, Anandaraj P, Hashikawa T, Nagao S, Arata A, Sakurai T, Yamamoto M, Ito M. Orexin-neuromodulated cerebellar circuit controls redistribution of arterial blood flows for defense behavior in rabbits. Proc Natl Acad Sci U S A. 2013;110:14124–31.
Rusyniak DE, Zaretsky DV, Zaretskaia MV, Dimicco JA. The role of orexin-1 receptors in physiologic responses evoked by microinjection of PgE2 or muscimol into the medial preoptic area. Neurosci Lett. 2011;498:162–6.
Arihara Z, Takahashi K, Murakami O, Totsune K, Sone M, Satoh F, Ito S, Hayashi Y, Sasano H, Mouri T. Orexin-A in the human brain and tumor tissues of ganglioneuroblastoma and neuroblastoma. Peptides. 2000;21(4):565–70.
Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, Tadayyon M, Clapham JC, Wilding J, Williams G. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes. 1999;48(11):2132–7.
Cai XJ, Evans ML, Lister CA, Leslie RA, Arch JR, Wilson S, Williams G. Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes. 2001;50(1):105–12.
Arihara Z, Takahashi K, Murakami O, Totsune K, Sone M, Satoh F, Ito S, Mouri T. Immunoreactive orexin-A in human plasma. Peptides. 2001;22(1):139–42.
Jöhren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142(8):3324–31.
Jöhren O, Brüggemann N, Dendorfer A, Dominiak P. Gonadal steroids differentially regulate the messenger ribonucleic acid expression of pituitary orexin type 1 receptors and adrenal orexin type 2 receptors. Endocrinology. 2003;144(4):1219–25.
Bornstein SR, Uhlmann K, Haidan A, Ehrhart-Bornstein M, Scherbaum WA. Evidence for a novel peripheral action of leptin as a metabolic signal to the adrenal gland: leptin inhibits cortisol release directly. Diabetes. 1997;46(7):1235–8.
Glasow A, Haidan A, Hilbers U, Breidert M, Gillespie J, Scherbaum WA, Chrousos GP, Bornstein SR. Expression of Ob receptor in normal human adrenals: differential regulation of adrenocortical and adrenomedullary function by leptin. J Clin Endocrinol Metab. 1998;83(12):4459–66.
Pralong FP, Roduit R, Waeber G, Castillo E, Mosimann F, Thorens B, Gaillard RC. Leptin inhibits directly glucocorticoid secretion by normal human and rat adrenal gland. Endocrinology. 1998;139(10):4264–8.
Glasow A, Bornstein SR. Leptin and the adrenal gland. Eur J Clin Investig. 2000;30(Suppl 3):39–45.
Ford GK, Al-Barazanji KA, Wilson S, Jones DN, Harbuz MS, Jessop DS. Orexin expression and function: glucocorticoid manipulation, stress, and feeding studies. Endocrinology. 2005;146(9):3724–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Imperatore, Palomba and Cristino declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hypertension and Metabolic Syndrome
Rights and permissions
About this article
Cite this article
Imperatore, R., Palomba, L. & Cristino, L. Role of Orexin-A in Hypertension and Obesity. Curr Hypertens Rep 19, 34 (2017). https://doi.org/10.1007/s11906-017-0729-y
Published:
DOI: https://doi.org/10.1007/s11906-017-0729-y