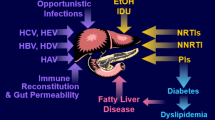
Abstract
Co-infection with HIV and hepatitis C (HCV) is common due to similar risk factors. Twenty-five to thirty percent of HIV+ patients are co-infected with HCV. HCV infection in HIV+ patients is associated with higher rates of fibrosis, progression to cirrhosis and decompensated liver disease, and liver-related mortality. The ultimate HCV treatment goal is viral eradication, or sustained virologic response (SVR) which results in decreased liver-related morbidity and mortality. Prior therapies were suboptimal in co-infected patients. However, the new HCV direct-acting antiviral agents provide excellent treatment options in co-infected patients with response rates and adverse events similar to the HCV mono-infected population. Drug interactions between HIV treatments and HCV treatments can be challenging and must be taken into consideration. To optimize outcomes, co-infected patients should be managed by experienced providers or in the setting of a collaborative multidisciplinary approach. This article will review the current treatment rationale and recommendations for HIV-HCV-co-infected patients.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS clinical trials group. Clin Infect Dis. 2002;34(6):831–7. doi:10.1086/339042.
Witt MD, Seaberg EC, Darilay A, Young S, Badri S, Rinaldo CR, et al. Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984–2011. Clin Infect Dis. 2013;57(1):77–84. doi:10.1093/cid/cit197.
van der Helm JJ, Prins M, del Amo J, Bucher HC, Chene G, Dorrucci M, et al. The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007. Aids. 2011;25(8):1083–91. doi:10.1097/QAD.0b013e3283471cce.
Fierer DS, Factor SH, Uriel AJ, Carriero DC, Dieterich DT, Mullen MP, et al. Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men—New York City, 2005-2010 (reprinted from MMWR, vol 60, pg 945-950, 2011). Jama-J Am Med Assoc. 2011;306(11):1194–6.
Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86(5):655–61. doi:10.2105/Ajph.86.5.655.
Kim AY, Chung RT. Coinfection with HIV-1 and HCV—a one-two punch. Gastroenterology. 2009;137(3):795–814. doi:10.1053/j.gastro.2009.06.040.
Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV cohort study. HIV Med. 2013;14(4):195–207. doi:10.1111/j.1468-1293.2012.01051.x.
Weber R. Liver-related deaths in persons infected with the human immunodeficiency virus—the D : A : D study. Arch Intern Med. 2006;166(15):1632–41. This paper describes the significantly increased risk of morbidity and mortality in patients with HIV and liver disease, especially in those with hepatitis C.
IDSA Aa. Recommendations for testing, managing, and treating hepatitis C. March, 10 2016. http://www.hcvguidelines.org/full-report-view. This website contains the AASLD/IDSA guidelines for the treatment of hepatitis C in all populations of patients. It is updated on a continuous basis by an expert panel.
Lin WY, Weinberg EM, Chung RT. Pathogenesis of accelerated fibrosis in HIV/HCV co-infection. J Infect Dis. 2013;207:S13–8. doi:10.1093/infdis/jis926.
Macias J, Marquez M, Tellez F, Merino D, Jimenez-Aguilar P, Lopez-Cortes LF, et al. Risk of liver decompensation among HIV/hepatitis C virus-coinfected individuals with advanced fibrosis: implications for the timing of therapy. Clin Infect Dis. 2013;57(10):1401–8. doi:10.1093/cid/cit537.
Curry MP. HIV and hepatitis C virus: special concerns for patients with cirrhosis. J Infect Dis. 2013;207:S40–4. doi:10.1093/infdis/jis763.
Chen TY, Ding EL, Seage GR, Kim AY. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis. 2009;49(10):1605–15. doi:10.1086/644771.
Ioannou GN, Bryson CL, Weiss NS, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection: are the data found in this sample applicable to other settings? Reply. Hepatology. 2013;57(6):2545. doi:10.1002/hep.26171.
Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New Engl J Med. 1998;338(13):853–60. doi:10.1056/Nejm199803263381301.
Swain MG, Lai MY, Shiffman ML, Cooksley WGE, Zeuzem S, Dieterich DT, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology. 2010;139(5):1593–601. doi:10.1053/j.gastro.2010.07.009.
Berenguer J, Alvarez-Pellicer J, Martin PM, Lopez-Aldeguer J, Von-Wichmann MA, Quereda C, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50(2):407–13. doi:10.1002/hep.23020.
Casado JL, Banon S, Quereda C, Moreno A, Elias MJP, Moreno S. Liver fibrosis regression after anti HCV therapy and the rate of death, liver-related death, liver-related complications and hospital admissions in HIV/HCV co-infected patients with cirrhosis. J Int Aids Soc. 2015;18. doi:10.7448/Ias.18.5.20364. This paper demonstrates that curing hepatitis C in co-infected patients is associated with decreased liver-related complications and mortality.
Berenguer J, Alvarez-Pellicer J, Carrero A, Von Wichmann MA, Lopez-Aldeguer J, Mallolas J, et al. Clinical effects of viral relapse after interferon plus ribavirin in patients co-infected with human immunodeficiency virus and hepatitis C virus. J Hepatol. 2013;58(6):1104–12.
Labarga P, Soriano V, Vispo ME, Pinilla J, Martin-Carbonero L, Castellares C, et al. Hepatotoxicity of antiretroviral drugs is reduced after successful treatment of chronic hepatitis C in HIV-infected patients. J Infect Dis. 2007;196(5):670–6. doi:10.1086/520092.
Nunez M, Miralles C, Berdun MA, Losada E, Aguirrebengoa K, Ocampo A, et al. Role of weight-based ribavirin dosing and extended duration of therapy in chronic hepatitis C in HIV-infected patients: the PRESCO trial. AIDS Res Hum Retroviruses. 2007;23(8):972–82. doi:10.1089/aid.2007.0011.
Chung RT, Umbleja T, Chen JY, Andersen JW, Butt AA, Sherman KE, et al. Extended therapy with pegylated interferon and weight-based ribavirin for HCV-HIV coinfected patients. HIV Clin Trials. 2012;13(2):70–82. doi:10.1310/hct1302-70.
Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-Garcia J, Lazzarin A, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. New Engl J Med. 2004;351(5):438–50. doi:10.1056/NEJMoa040842.
Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. New Engl J Med. 2004;351(5):451–9. doi:10.1056/NEJMoa032653.
Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients—a randomized controlled trial. Jama-J Am Med Assoc. 2004;292(23):2839–48. doi:10.1001/jama.292.23.2839.
Benito JM, Sanchez-Parra C, Maida I, Aguilera A, Rallon NI, Rick F, et al. Triple combination therapy for hepatitis C with telaprevir exhibits greater early antiviral activity than with boceprevir. Antivir Ther. 2013;18(5):709–15. doi:10.3851/Imp2614.
Cachay ER, Wyles DL, Torriani FJ, Ballard C, Colwell B, Lin JC, et al. High incidence of serious adverse events in HIV-infected patients treated with a telaprevir-based hepatitis C virus treatment regimen. Aids. 2013;27(18):2893–7. doi:10.1097/01.aids.0000432466.15885.14.
Cotte L BJ, Lascoux-Combe C, et al. High early virological response with telaprevir-pegylated interferon-ribavirin in treatment-experienced HCV genotype 1/HIV coinfected patients: ANRS HC26 TelapreVIH study. CROI 2013. Atlanta, GA. Abstract. March 3-6 2013.
Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, et al. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis (virus genotype 1) in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13(7):597–605. doi:10.1016/S1473-3099(13)70149-X.
Dieterich D, Rockstroh JK, Orkin C, et al. Simeprevir (TMC435) plus PegIFN/ribavirin in HCV genotype-1/HIV-1 coinfection (study C212).[Croi abstract 24] in special issue: abstracts from the 2014 conference on retroviruses and opportunistic infections. Top Antivir Med. 2014;22(e-1):34.
Sulkowski MS. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. New Engl J Med. 2014;370(15):1469. doi:10.1056/NEJMx140017.
Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. New Engl J Med. 2014;370(3):222–32. doi:10.1056/NEJMoa1306227.
Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. New Engl J Med. 2014;370(21):1973–82. doi:10.1056/NEJMoa1402869.
Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. New Engl J Med. 2014;370(21):1983–92. doi:10.1056/NEJMoa1402338.
Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. New Engl J Med. 2014;370(20):1889–98. doi:10.1056/NEJMoa1402454.
Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. New Engl J Med. 2014;370(16):1483–93. doi:10.1056/NEJMoa1316366.
Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. New Engl J Med. 2014;370(20):1879–88. doi:10.1056/NEJMoa1402355.
Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. New Engl J Med. 2015;373(8):705–13. doi:10.1056/NEJMoa1501315. This paper showed the data from treating HIV-HCV co-infected patients with the all-oral HCV regimen of ledipasvir and sofosbuvir. The overall sustained virologic response rate for these HCV genotype 1 and 4 patients was 96 %.
Khatri A WT, Wang H et al. Drug-drug interactions of the direct-acting antiviral regimen of ABT-450/r, ombitasvir, and dasabuvir with emtricabine + tenofovir, raltegravir, rilpivirine, and efavirenz. [Abstract 483.] 54th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Washington, DC. September 5-9,2014.
Rockstroh JK, Nelson M, Katlama C. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial (vol 2, pg e319, 2015). Lancet Hiv. 2015;2(10):E416.
Sulkowski MS. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection (vol 312, pg 353, 2014). Jama-J Am Med Assoc. 2014;312(18):1932.
Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, et al. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet. 2015;385(9973):1098–106. doi:10.1016/S0140-6736(14)62483-1.
Wyles DL, Ruane PJ, Sulkowski MS, Dieterich D, Luetkemeyer A, Morgan TR, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. New Engl J Med. 2015;373(8):714–25. doi:10.1056/NEJMoa1503153. This paper showed the data from treating HIV-HCV co-infected patients with the all-oral HCV regimen of daclatasvir and sofosbuvir. The overall sustained virologic response rate for these HCV genotype 1–4 patients was 97 %.
Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. New Engl J Med. 2013;368(20):1878–87. doi:10.1056/NEJMoa1214853.
Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. April8, 2015;1-288. April 8, 2015. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
Harvoni prescribing information http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf.
Osinusi A, Townsend K, Kohli A, Nelson A, Seamon C, Meissner EG, et al. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. Jama-J Am Med Assoc. 2015;313(12):1232–9. doi:10.1001/jama.2015.1373.
Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: a phase 3 study (OPTIMIST-2). Hepatology. 2015. doi:10.1002/hep.28422.
Kirby BMA, Rossi S, et al. No clinically significant pharmacokinetic drug interactions between sofosbuvir (GS-7977) and HIV antiretrovirals atripla, rilpivirine, darunavir/ritonavir, or raltegravir in healthy volunteers. Boston: 63rd Annual Meeting of the American Association of the Study of Liver Diseases (AASLD); 2012.
Sofosbuvir prescribing information http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/sovaldi/sovaldi_pi.pdf.
German P PP, West S, et al. Drug interactions between direct acting anti-HCV antivirals sofosbuvir and ledipasvir and HIV antiretrovirals. [Abstract 06.] 15th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. Washington, DC. May,19-21,2014.
Garrison K CJ, Pang P, et al. Drug Interactions between anti HCV Antivirals Ledipasvir/Sofosbuvir and Integrase Strand Transfer Inhibitor-Based Regimens [Abstract 71]. 16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. Washington, DC. May 26-28, 2015.
Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV medicine association of the infectious diseases society of America. Clin Infect Dis. 2014;59(9):e96–138. doi:10.1093/cid/ciu617.
Viekira pak prescribing information http://www.rxabbvie.com/pdf/viekirapak_pi.pdf.
Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA. 2015;313(12):1223–31. doi:10.1001/jama.2015.1328.
Khatri A TR, Zhao W, et al. Drug-drug interactions of ombitasvir/paritaprevir/r plus dasabuvir with dolutegravir or abacavir plus lamivudine. [Abstract 57.] 16th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy. Washington, DC. May 26-28, 2015.
Declatasvir full prescribing information http://packageinserts.bms.com/pi/pi_daklinza.pdf.
Bifano M, Hwang C, Oosterhuis B, Hartstra J, Grasela D, Tiessen R, et al. Assessment of pharmacokinetic interactions of the HCV NS5A replication complex inhibitor daclatasvir with antiretroviral agents: ritonavir-boosted atazanavir, efavirenz and tenofovir. Antivir Ther. 2013;18(7):931–40. doi:10.3851/Imp2674.
Gandhi Y AR, Wang R, et al. Assessment of drug-drug interactions between daclatasvir and darunavir/ritonavir or lopinavir/ritonavir. [Abstract 80.] 16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. Washington, DC. Mat 26-28, 2015.
Song I JF, Zong J, et al. Evaluation and drug interactions between dolutegravir and daclatasvir in healthy volunteers. [Abstract 79.] 16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. Washington, DC. May 26-28,2015.
Zepatier full prescribing information https://www.merck.com/product/usa/pi_circulars/z/zepatier/zepatier_pi.pdf
Fleischer R, Boxwell D, Sherman KE. Nucleoside analogues and mitochondrial toxicity. Clin Infect Dis. 2004;38(8):E79–80. doi:10.1086/383151.
Alvarez D, Dieterich DT, Brau N, Moorehead L, Ball L, Sulkowski MS. Zidovudine use but not weight-based ribavirin dosing impacts anaemia during HCV treatment in HIV-infected persons. J Viral Hepatitis. 2006;13(10):683–9. doi:10.1111/j.1365-2893.2006.00749.x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
PD declares that he has no conflicts of interest. MKO reports grants from and advisory board membership with Gilead, outside the submitted work. SMC reports consultancy and speaker’s bureau participation for Gilead; grants from and consultancy and speaker’s bureau participation for Bristol-Myers Squibb; and grants from and consultancy and speaker’s bureau participation for Abbvie, outside the submitted work.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and were in compliance with all applicable ethical standards.
Additional information
This article is part of the Topical Collection on Hepatitis C
Rights and permissions
About this article
Cite this article
Davitkov, P., Osborn, M.K. & Cohen, S.M. Hepatitis C Management in Patients with Hepatitis C and HIV Co-infection. Curr Hepatology Rep 15, 158–166 (2016). https://doi.org/10.1007/s11901-016-0307-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-016-0307-9