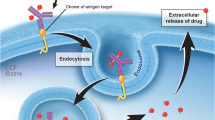
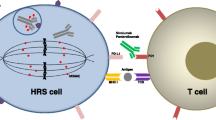
Abstract
Evolution of cancer therapeutics has resulted in the development of agents with varying mechanisms of selective target inhibition. One such therapeutic approach is utilizing antibody-drug conjugates (ADCs), the combination of a cytotoxic agent linked with a monoclonal antibody, to achieve localization of the target and internalization of the cytotoxic agent in order to maximize efficacy with fewer toxicities. This review focuses on CD30 as a therapeutic target and the development and clinical activity of the ADC brentuximab vedotin in Hodgkin lymphoma (HL) and other B cell lymphomas.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Agarwal P, Bertozzi CR. Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug Chem. 2015;26(2):176–92. doi:10.1021/bc5004982.
Elias DJ, Kline LE, Robbins BA, Johnson Jr HC, Pekny K, Benz M, et al. Monoclonal antibody KS1/4-methotrexate immunoconjugate studies in non-small cell lung carcinoma. Am J Respir Crit Care Med. 1994;150(4):1114–22. doi:10.1164/ajrccm.150.4.7921445.
Arcaini L, Merli M, Passamonti F, Rizzi S, Ferretti V, Rattotti S, et al. Validation of follicular lymphoma international prognostic index 2 (FLIPI2) score in an independent series of follicular lymphoma patients. Br J Haematol. 2010;149(3):455–7. doi:10.1111/j.1365-2141.2009.08065.x.
FDA approves Adcetris to treat two types of lymphoma. 2011.
Durkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin’s disease. Cell. 1992;68(3):421–7.
Muta H, Podack ER. CD30: from basic research to cancer therapy. Immunol Res. 2013;57(1–3):151–8. doi:10.1007/s12026-013-8464-1.
Wahl AF, Klussman K, Thompson JD, Chen JH, Francisco LV, Risdon G, et al. The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin’s disease. Cancer Res. 2002;62(13):3736–42.
Borchmann P, Treml JF, Hansen H, Gottstein C, Schnell R, Staak O, et al. The human anti-CD30 antibody 5F11 shows in vitro and in vivo activity against malignant lymphoma. Blood. 2003;102(10):3737–42. doi:10.1182/blood-2003-02-0515.
Schwab U, Stein H, Gerdes J, Lemke H, Kirchner H, Schaadt M, et al. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin’s disease and a subset of normal lymphoid cells. Nature. 1982;299(5878):65–7.
Gerdes J, Schwarting R, Stein H. High proliferative activity of Reed Sternberg associated antigen Ki-1 positive cells in normal lymphoid tissue. J Clin Pathol. 1986;39(9):993–7.
Ralfkiaer E, Bosq J, Gatter KC, Schwarting R, Gerdes J, Stein H, et al. Expression of a Hodgkin and Reed-Sternberg cell associated antigen (Ki-1) in cutaneous lymphoid infiltrates. Arch Dermatol Res. 1987;279(5):285–92.
Telford WG, Nam SY, Podack ER, Miller RA. CD30-regulated apoptosis in murine CD8 T cells after cessation of TCR signals. Cell Immunol. 1997;182(2):125–36. doi:10.1006/cimm.1997.1228.
Bowen MA, Lee RK, Miragliotta G, Nam SY, Podack ER. Structure and expression of murine CD30 and its role in cytokine production. J Immunol. 1996;156(2):442–9.
Chiarle R, Podda A, Prolla G, Podack ER, Thorbecke GJ, Inghirami G. CD30 overexpression enhances negative selection in the thymus and mediates programmed cell death via a Bcl-2-sensitive pathway. J Immunol. 1999;163(1):194–205.
DeYoung AL, Duramad O, Winoto A. The TNF receptor family member CD30 is not essential for negative selection. J Immunol. 2000;165(11):6170–3.
Wiley SR, Goodwin RG, Smith CA. Reverse signaling via CD30 ligand. J Immunol. 1996;157(8):3635–9.
Falini B, Pileri S, Pizzolo G, Durkop H, Flenghi L, Stirpe F, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85(1):1–14.
Stein H, Foss HD, Durkop H, Marafioti T, Delsol G, Pulford K, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96(12):3681–95.
Horie R, Watanabe T. CD30: expression and function in health and disease. Semin Immunol. 1998;10(6):457–70. doi:10.1006/smim.1998.0156.
Chiarle R, Podda A, Prolla G, Gong J, Thorbecke GJ, Inghirami G. CD30 in normal and neoplastic cells. Clin Immunol. 1999;90(2):157–64. doi:10.1006/clim.1998.4636.
Gardner LJ, Polski JM, Evans HL, Perkins SL, Dunphy CH. CD30 expression in follicular lymphoma. Arch Pathol Lab Med. 2001;125(8):1036–41. doi:10.1043/0003-9985(2001)125<1036:CEIFL>2.0.CO;2.
Latza U, Foss HD, Durkop H, Eitelbach F, Dieckmann KP, Loy V, et al. CD30 antigen in embryonal carcinoma and embryogenesis and release of the soluble molecule. Am J Pathol. 1995;146(2):463–71.
Ferreiro JA. Ber-H2 expression in testicular germ cell tumors. Hum Pathol. 1994;25(5):522–4.
Hittmair A, Rogatsch H, Hobisch A, Mikuz G, Feichtinger H. CD30 expression in seminoma. Hum Pathol. 1996;27(11):1166–71.
Hu S, Xu-Monette ZY, Balasubramanyam A, Manyam GC, Visco C, Tzankov A, et al. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2013;121(14):2715–24. doi:10.1182/blood-2012-10-461848.
Campuzano-Zuluaga G, Cioffi-Lavina M, Lossos IS, Chapman-Fredricks JR. Frequency and extent of CD30 expression in diffuse large B-cell lymphoma and its relation to clinical and biologic factors: a retrospective study of 167 cases. Leuk Lymphoma. 2013;54(11):2405–11. doi:10.3109/10428194.2013.778407.
Slack GW, Steidl C, Sehn LH, Gascoyne RD. CD30 expression in de novo diffuse large B-cell lymphoma: a population-based study from British Columbia. Br J Haematol. 2014;167(5):608–17. doi:10.1111/bjh.13085.
Wang XJ, Seegmiller AC, Reddy NM, Li S. CD30 expression and its correlation with MYC rearrangement in de novo diffuse large B-cell lymphoma. Eur J Haematol. 2016;97(1):39–47. doi:10.1111/ejh.12680.
Gruss HJ, Pinto A, Gloghini A, Wehnes E, Wright B, Boiani N, et al. CD30 ligand expression in nonmalignant and Hodgkin’s disease-involved lymphoid tissues. Am J Pathol. 1996;149(2):469–81.
Matsumoto K, Terakawa M, Miura K, Fukuda S, Nakajima T, Saito H. Extremely rapid and intense induction of apoptosis in human eosinophils by anti-CD30 antibody treatment in vitro. J Immunol. 2004;172(4):2186–93.
Hartmann F, Renner C, Jung W, Deisting C, Juwana M, Eichentopf B, et al. Treatment of refractory Hodgkin’s disease with an anti-CD16/CD30 bispecific antibody. Blood. 1997;89(6):2042–7.
Hartmann F, Renner C, Jung W, Pfreundschuh M. Anti-CD16/CD30 bispecific antibodies as possible treatment for refractory Hodgkin’s disease. Leuk Lymphoma. 1998;31(3–4):385–92. doi:10.3109/10428199809059232.
Borchmann P, Schnell R, Fuss I, Manzke O, Davis T, Lewis LD, et al. Phase 1 trial of the novel bispecific molecule H22xKi-4 in patients with refractory Hodgkin lymphoma. Blood. 2002;100(9):3101–7. doi:10.1182/blood-2001-12-0295.
Falini B, Bolognesi A, Flenghi L, Tazzari PL, Broe MK, Stein H, et al. Response of refractory Hodgkin’s disease to monoclonal anti-CD30 immunotoxin. Lancet. 1992;339(8803):1195–6.
Schnell R, Staak O, Borchmann P, Schwartz C, Matthey B, Hansen H, et al. A Phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphoma. Clin Cancer Res. 2002;8(6):1779–86.
Schnell R, Borchmann P, Staak JO, Schindler J, Ghetie V, Vitetta ES, et al. Clinical evaluation of ricin A-chain immunotoxins in patients with Hodgkin’s lymphoma. Ann Oncol. 2003;14(5):729–36.
Schnell R, Dietlein M, Staak JO, Borchmann P, Schomaecker K, Fischer T, et al. Treatment of refractory Hodgkin’s lymphoma patients with an iodine-131-labeled murine anti-CD30 monoclonal antibody. J Clin Oncol. 2005;23(21):4669–78. doi:10.1200/JCO.2005.09.098.
Ansell SM, Horwitz SM, Engert A, Khan KD, Lin T, Strair R, et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25(19):2764–9. doi:10.1200/JCO.2006.07.8972.
Bartlett N, Younes A. M C. Phase I study of SGN-30, a chimeric monoclonal antibody (mAb), in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2002;100:362a–3a.
Bartlett NL, Younes A, Carabasi MH, Forero A, Rosenblatt JD, Leonard JP, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111(4):1848–54. doi:10.1182/blood-2007-07-099317.
Forero-Torres A, Leonard JP, Younes A, Rosenblatt JD, Brice P, Bartlett NL, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146(2):171–9. doi:10.1111/j.1365-2141.2009.07740.x.
Cerveny CG, Law CL, McCormick RS, Lenox JS, Hamblett KJ, Westendorf LE, et al. Signaling via the anti-CD30 mAb SGN-30 sensitizes Hodgkin’s disease cells to conventional chemotherapeutics. Leukemia. 2005;19(9):1648–55. doi:10.1038/sj.leu.2403884.
Heuck F, Ellermann J, Borchmann P, Rothe A, Hansen H, Engert A, et al. Combination of the human anti-CD30 antibody 5F11 with cytostatic drugs enhances its antitumor activity against Hodgkin and anaplastic large cell lymphoma cell lines. J Immunother. 2004;27(5):347–53.
Blum KA, Jung SH, Johnson JL, Lin TS, Hsi ED, Lucas DM, et al. Serious pulmonary toxicity in patients with Hodgkin’s lymphoma with SGN-30, gemcitabine, vinorelbine, and liposomal doxorubicin is associated with an FcgammaRIIIa-158 V/F polymorphism. Ann Oncol. 2010;21(11):2246–54. doi:10.1093/annonc/mdq211.
Sutherland MS, Sanderson RJ, Gordon KA, Andreyka J, Cerveny CG, Yu C, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281(15):10540–7. doi:10.1074/jbc.M510026200.
Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity [Abstract]. Blood. 2003;102(4):1458–65. doi:10.1182/blood-2003-01-0039.
Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–70. doi:10.1158/1078-0432.CCR-04-0789.
Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res. 2010;16(3):888–97. doi:10.1158/1078-0432.CCR-09-2069.
Oflazoglu E, Kissler KM, Sievers EL, Grewal IS, Gerber HP. Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br J Haematol. 2008;142(1):69–73. doi:10.1111/j.1365-2141.2008.07146.x.
Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–21. doi:10.1056/NEJMoa1002965.
Fanale MA, Forero-Torres A, Rosenblatt JD, Advani RH, Franklin AR, Kennedy DA, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res. 2012;18(1):248–55. doi:10.1158/1078-0432.CCR-11-1425.
Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006;24(10):1633–42. doi:10.1200/JCO.2005.04.0543.
Forero-Torres A, Fanale M, Advani R, Bartlett NL, Rosenblatt JD, Kennedy DA, et al. Brentuximab vedotin in transplant-naive patients with relapsed or refractory hodgkin lymphoma: analysis of two phase I studies. Oncologist. 2012;17(8):1073–80. doi:10.1634/theoncologist.2012-0133.
Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–9. doi:10.1200/JCO.2011.38.0410. This pivotal phase II open-label trial lead to accelerated FDA approval of brentuximab vedotin in relapsed/refractory Hodgkin disease after autologous transplant. Objective responses were noted in 75% with complete remissions in 34% of the patients at a median follow-up of 1.5 years.
Wagner-Johnston ND, Bartlett NL, Cashen A, Berger JR. Progressive multifocal leukoencephalopathy in a patient with Hodgkin lymphoma treated with brentuximab vedotin. Leuk Lymphoma. 2012;53(11):2283–6. doi:10.3109/10428194.2012.676170.
Carson KR, Newsome SD, Kim EJ, Wagner-Johnston ND, von Geldern G, Moskowitz CH, et al. Progressive multifocal leukoencephalopathy associated with brentuximab vedotin therapy: a report of 5 cases from the Southern Network on Adverse Reactions (SONAR) project. Cancer. 2014;120(16):2464–71. doi:10.1002/cncr.28712.
von Geldern G, Pardo CA, Calabresi PA, Newsome SD. PML-IRIS in a patient treated with brentuximab. Neurology. 2012;79(20):2075–7. doi:10.1212/WNL.0b013e3182749f17.
Howlader NNA, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; 2016.
Kuruvilla JKA, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011;117(16):4208–17. doi:10.1182/blood-2010-09-288373.
Gopal AK, Chen R, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(8):1236–43. doi:10.1182/blood-2014-08-595801.
Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016. doi:10.1182/blood-2016-02-699850.
Bartlett NL, Chen R, Fanale MA, Brice P, Gopal A, Smith SE, et al. Retreatment with brentuximab vedotin in patients with CD30-positive hematologic malignancies. J Hematol Oncol. 2014;7:24. doi:10.1186/1756-8722-7-24.
Chen R, Palmer JM, Martin P, Tsai N, Kim Y, Chen BT, et al. Results of a multicenter phase II trial of brentuximab vedotin as second-line therapy before autologous transplantation in relapsed/refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2015;21(12):2136–40. doi:10.1016/j.bbmt.2015.07.018.
LaCasce ABG, Sawas A, Caimi P, Agura E, Matous J, Ansell S, et al. Brentuximab vedotin plus bendamustine: a highly active salvage treatment regimen for patients with relapsed or refractory Hodgkin lymphoma [abstract]. Blood. 2015;126:3982.
Moskowitz AJ, Schoder H, Yahalom J, McCall SJ, Fox SY, Gerecitano J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosfamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin’s lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284–92. doi:10.1016/S1470-2045(15)70013-6. Interim analysis of salvage therapy with brentuximab vedotin showed complete remissions prior to autologous transplants when used in combination with other salvage regimens.
Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–62. doi:10.1016/S0140-6736(15)60165-9.
Younes A, Connors JM, Park SI, Fanale M, O’Meara MM, Hunder NN, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348–56. doi:10.1016/S1470-2045(13)70501-1.
Connors JAS, Park S, Fanale MA, Younes A. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed advanced stage Hodgkin lymphoma: long term outcomes [Abstract]. San Francisco: American Society of Hematology; 2014. Long term results of phase I open-label dose escalation study of BV in combination with standard chemotherapy (ABVD) in frontline setting has shown increased pulmonary toxicity in the combination. The study was modified to exclude bleomycin and durable responses have been noted in 96% with 3 year overall survival rate of 100%. Phase III trial (ECHELON-1) comparing efficacy and safety of this combination with standard ABVD is ongoing (clinicaltrials.gov; NCT01712490).
Forero-Torres AHB, Sharman JP, Friedberg JW, Berkowitz MJ, Fintel W, Chen R, et al. Brentuximab vedotin monotherapy and in combination with dacarbazine in frontline treatment of Hodgkin lymphoma in patients aged 60 years and above: interim results of a phase 2 study [Abstract]. Blood. 2014;124:294.
Moskowitz AJ, Hamlin Jr PA, Perales MA, Gerecitano J, Horwitz SM, Matasar MJ, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31(4):456–60. doi:10.1200/JCO.2012.45.3308.
Yasenchak CF-TA, Cline-Burkhardt V, Bordoni R, Patel-Donnelly D, Flynn P, Chen R, et al. Brentuximab vedotin in combination with dacarbazine or bendamustine for frontline treatment of Hodgkin lymphoma in patients aged 60 years and above: interim results of a multi-cohort phase 2 study. Orlando, FL: American Society of Hematology; 2015.
Hill BME, Smith M, Hsi E. CD30 immunohistochemical expression in diffuse large B-cell lymphoma is associated with decreased overall survival and the non-germinal center molecular subtype [Abstract]. Blood. 2013;122:4318.
Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125(9):1394–402. doi:10.1182/blood-2014-09-598763.
NCCN guidelines In: https://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.
Bartlett NSM, Advani R, Feldman T, Savage K, Palanca-Wessels M, Siddiqi T. Brentuximab vedotin monotherapy in DLBCL patients with undetectable CD30: preliminary results from a phase 2 study. San Francisco, CA: American Society of Hematology; 2014.
Yasenchak CFC, Budde L, Ansell S, Advani R, Holkova B, Halwani A, et al. Brentuximab vedotin in combination with RCHOP as front-line therapy in patients with DLBCL: interim results from a phase 2 study. Blood. 2014;124:1745.
Yasenchak CHA, Advani R, Ansell S, Budde L, Burke J, Farber C, et al. Brentuximab vedotin with RCHOP as frontline therapy in patients with high-intermediate/high-risk diffuse large B cell lymphoma (DLBCL): results from an ongoing phase 2 study [Abstract]. Orlando: American Society of Hematology; 2015. A Phase II trial of combining BV with R-CHOP as frontline treatment in high and high-intermediate risk DLBCL showed higher CR rates in CD30 positive patients with overall response rates of 97% and CR in 80% in interim analysis.
Ward JTJ, Luo J, Wagner-Johnston N, Cashen A, Fehniger T, Bartlett N. A phase I trial of brentuximab vedotin in combination with lenalidomide in relapsed or refractory diffuse large B-cell lymphoma [Abstract]. Orlando: American Society of Hematology; 2015. A phase I trial of lenalidomide in combination with brentuximab vedotin in relapsed/refractory DLBCL showed overall response rate of 53% with complete remissions noted in 41% in interim analysis.
Zheng W, Medeiros LJ, Young KH, Goswami M, Powers L, Kantarjian HH, et al. CD30 expression in acute lymphoblastic leukemia as assessed by flow cytometry analysis. Leuk Lymphoma. 2014;55(3):624–7. doi:10.3109/10428194.2013.820293.
Fathi AT, Preffer FI, Sadrzadeh H, Ballen KK, Amrein PC, Attar EC, et al. CD30 expression in acute myeloid leukemia is associated with FLT3-internal tandem duplication mutation and leukocytosis. Leuk Lymphoma. 2013;54(4):860–3. doi:10.3109/10428194.2012.728596.
Fathi AT, Chen YB. CD30: a new target for ALL? Leuk Lymphoma. 2014;55(3):478–9. doi:10.3109/10428194.2013.823496.
Zheng W, Medeiros LJ, Hu Y, Powers L, Cortes JE, Ravandi-Kashani F, et al. CD30 expression in high-risk acute myeloid leukemia and myelodysplastic syndromes. Clin Lymphoma Myeloma Leuk. 2013;13(3):307–14. doi:10.1016/j.clml.2012.12.006.
Schober T, Framke T, Grosshennig A, Klein C, Kreipe H, Maecker-Kolhoff B. CD30 in pediatric post-transplant lymphoproliferative disease after solid organ transplant: characterization of a new therapeutic target. Leuk Lymphoma. 2015;56(3):832–3. doi:10.3109/10428194.2014.941837.
Borate U, Mehta A, Reddy V, Tsai M, Josephson N, Schnadig I. Treatment of CD30-positive systemic mastocytosis with brentuximab vedotin. Leuk Res. 2016;44:25–31. doi:10.1016/j.leukres.2016.02.010.
Gandhi MMS, Smith SM, Nabhan C, Evens AM, Winter JN, Gordon LI, et al. Brentuximab vedotin (BV) plus rituximab (R) as frontline therapy for patients (Pts) with Epstein Barr virus (EBV)+ and/or CD30+ lymphoma: phase I results of an ongoing phase I-II study. San Francisco, CA: American Society of Hematology; 2014.
Svoboda JSL, Nasta S, Landsburg D, Mato A, Pro M, Barta S, et al. Brentuximab vedotin in combination with multi-agent chemotherapy is well tolerated and shows promising activity as frontline treatment for primary mediastinal B-cell lymphoma. Orlando, FL: American Society of Hematology; 2015.
Federico MPE, Merli F, Luminari S, Chauvie S, Pellegrini C, Marcheselli L, et al. A pilot phase II study with brentuximab vedotin followed by ABVD in patients with previously untreated Hodgkin lymphoma: a preliminary report. J Clin Oncol. 2014;32:5s2014. suppl; abstr 8507.
Abramson JAJ, LaCasce A, Redd R, Barnes J, Sokol L, Joyce R, et al. Brentuximab vedotin plus AVD for non-bulky limited stage Hodgkin lymphoma: a phase II trial. J Clin Oncol. 2015;33:8505.
Gopal AK, Bartlett NL, Forero-Torres A, Younes A, Chen R, Friedberg JW, et al. Brentuximab vedotin in patients aged 60 years or older with relapsed or refractory CD30-positive lymphomas: a retrospective evaluation of safety and efficacy. Leuk Lymphoma. 2014;55(10):2328–34. doi:10.3109/10428194.2013.876496.
Sweetenham JWJ, Nadamanee A, Masszi T, Agura E, Holowiecki J, Abidi M, et al. Updated efficacy and safety data from the AETHERA trial of consolidation with brentuximab vedotin after autologous stem cell transplant (ASCT) in Hodgkin lymphoma patients at high risk of relapse [Abstract]. Biol Blood Marrow Transplant. 2016;22(3):S36–7. doi:10.1016/j.bbmt.2015.11.315.
Viviani S GA, Dalto S, Dodero A, Farina L, Bolis S, Rigacci L, Zallio F, Busca A, Bruno B, Mordini N, Parvis G, Corradini P. Brentuximab vedotin (BV) an effective treatment for transplant ineligible patients with relapsed/refractory (R/R) Hodgkin lymphoma (HL). European Hematology Association; June 12: EHA Learning Center. Jun 12, 2015; 100326; 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Geetika Bhatt declares no potential conflicts of interest. Kami Maddocks and Beth Christian have research support from Seattle Genetics, Celgene, and Bristol-Myers Squibb.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on T-Cell and Other Lymphoproliferative Malignancies
Rights and permissions
About this article
Cite this article
Bhatt, G., Maddocks, K. & Christian, B. CD30 and CD30-Targeted Therapies in Hodgkin Lymphoma and Other B cell Lymphomas. Curr Hematol Malig Rep 11, 480–491 (2016). https://doi.org/10.1007/s11899-016-0345-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-016-0345-y