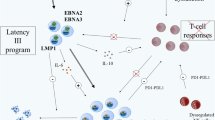
Opinion statement
Post-transplant lymphoproliferative disorder (PTLD) is one of the most common neoplasms seen after solid organ and hematopoietic stem cell transplantation, and is associated with significant morbidity and mortality. The pathogenesis is related to post-transplant immunosuppression and EBV infection. Prevention of PTLD depends upon judicious use of immunosuppression and serial EBV monitoring. Preemptive therapy consists of reduction of immunosuppression, antiviral medications, and single-agent rituximab. There are no randomized phase III trials on PTLD treatment, so current management guidelines are largely based on recent phase II trials, single-institution retrospective studies, and expert opinion. Management of PTLD is dependent upon its subtypes. Early-type and polymorphic PTLD generally respond to reduction of immunosuppression and rituximab monotherapy, whereas monomorphic PTLD often requires additional concurrent or sequential use of chemotherapy. For rare subtypes of PTLD, standard-of-care guidelines for de novo lymphomas are recommended. Surgical resection or radiotherapy may be used as adjunctive therapy depending on the extent of disease. Non-chemotherapy options such as adoptive T cell therapy have shown promising efficacy and must be explored further. Despite progress in the last decade, overall survival rates continue to be low in published series. This review highlights the need for prospective randomized trials incorporating novel agents to improve outcomes in PTLD.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Parker A, Bowles K, Bradley JA. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients—BCSH and BTS guidelines. Br J Haematol. 2010;149(5):675–92.
•• Ligeti K, Müller LP, Müller-Tidow C, Weber T. Risk factors, diagnosis, and management of posttransplant lymphoproliferative disorder: improving patient outcomes with a multidisciplinary treatment approach. Transplant Res Risk Manag. 2017;9:1–4. This 2017 review is an extensive and comprehensive summary of SOT- and HSCT-related PTLDs and includes a summary of all studied therapies, including newer adoptive immunotherapy, as well as therapies for rare subtypes of PTLD.
••Dierickx D, Tousseyn T, Gheysens O. How I treat posttransplant lymphoproliferative disorders. Blood. 2015;126:2274–83. https://doi.org/10.1182/blood-2015-05-615872.This 2015 “How I Treat” review in Blood presents several illustrative cases as the foundation for a detailed discussion of the diagnosis and staging of PTLDs. In addition, there are sections focusing on EBV viral load in the diagnostic criteria of PTLD and EBV-negative PTLD.
• Petrara MR, Giunco S, Serraino D, Dolcetti R, de Rossi A. Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment. Cancer Lett. 2015;369:37–44. https://doi.org/10.1016/j.canlet.2015.08.007.This 2015 review provides a detailed summary of the pathogenesis of PTLD and how pathogenesis informs therapy.
Uhlin M, Wikell H, Sundin M, Blennow O, Maeurer M, Ringden O, et al. Risk factors for Epstein-Barr virus-related post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014;99:346–52. https://doi.org/10.3324/haematol.2013.087338.
Preiksaitis JK, Keay S. Diagnosis and management of posttransplant lymphoproliferative disorder in solid-organ transplant recipients. Clin Infect Dis. 2001;33(1):S38–46. https://doi.org/10.1086/320903.
Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222–30.
Davis CL, Wood BL, Sabath DE, Joseph JS, Stehman-Breen C, Broudy VC. Interferon-alpha treatment of posttransplant lymphoproliferative disorder in recipients of solid organ transplants. Transplantation. 1998;66(12):1770–9.
Evens AM, David KA, Helenowski I, Nelson B, Kaufman D, Kircher SM, et al. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28(6):1038–46.
• Nijland ML, Kersten MJ, Pals ST, et al. Epstein-Barr virus-positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplant Direct. 2016;2(1):e48. https://doi.org/10.1097/TXD.0000000000000557.This 2016 review of PTLD provides a comprehensive summary of the pathophysiology, risk factors, subtypes, prevention, and therapies.
Luskin MR, Heil DS, Tan KS, Choi S, Stadtmauer EA, Schuster SJ, et al. The impact of EBV status on characteristics and outcomes of posttransplantation lymphoproliferative disorders. Am J Transplant. 2015;15(10):2665–73.
Dierickx D, Tousseyn T, Sagaert X, Fieuws S, Wlodarska I, Morscio J, et al. Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. 2013;54(11):2433–40.
Allen UD, Preiksaitis JK. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):107–20. https://doi.org/10.1111/ajt.12104.
Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol. 2012;9(9):510–9. https://doi.org/10.1038/nrclinonc.2012.111.
Armitage JM, Kormos RL, Stuart RS, et al. Postransplant lymphoproliferative disease in thoracic organ transplant patients: ten years of cyclosporine-based immunosuppression. J Heart Lung Transplant. 1991;10:877–87.
Leyssens A, Dierickx D, Verbeken EK, et al. Post-transplant lymphoproliferative disease in lung transplantation: a nested case-control study. First published: 6 April 2017 https://doi.org/10.1111/ctr.12983.
• Bishnoi R, Bajwa R, Franke AJ, et al. Post-transplant lymphoproliferative disorder (PTLD): single institutional experience of 141 patients. Exp Hematol Oncol. 2017;6:26. A 2017 single-center study of 141 patients with PTLD presents a very recent description of improving survival in PTLD and highlights the mixed evidence on rituximab monotherapy in PTLD.
Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, van der Velden W, et al. Response to rituximab-based therapy and risk factor analysis in Epstein Barr virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2013;57(6):794–802.
Sanz J, Arango M, Senent L, Jarque I, Montesinos P, Sempere A, et al. EBV-associated post-transplant lymphoproliferative disorder after umbilical cord blood transplantation in adults with hematological diseases. Bone Marrow Transplant. 2014;49(3):397–402.
Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JAH, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108(8):2874–80.
Ibrahim HAH, Naresh KN. Posttransplant lymphoproliferative disorders. Adv Hematol. 2012;2012:1–11. https://doi.org/10.1155/2012/230173.
Pascual J. Post-transplant lymphoproliferative disorder—the potential of proliferation signal inhibitors. Nephrol Dial Transplant. 2007;22(Suppl 1):i27–35.
Merlo A, Turrini R, Dolcetti R, Martorelli D, Muraro E, Comoli P, et al. The interplay between Epstein-Barr virus and the immune system: a rationale for adoptive cell therapy of EBV-related disorders. Haematologica. 2010;95:1769–77. https://doi.org/10.3324/haematol.2010.023689.
Gulley ML, Tang W. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin Microbiol Rev. 2010;23(2):350–66.
Mañez R, Breinig MC, Linden P, Wilson J, Torre-Cisneros J, Kusne S, et al. Posttransplant lymphoproliferative disease in primary Epstein–Barr virus infection after liver transplantation: the role of cytomegalovirus disease. J Infect Dis. 1997;176:1462–7.
Landgren O, Gilbert ES, Rizzo JD, Socie G, Banks PM, Sobocinski KA, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113(20):4992–5001.
Riva G, Luppi M, Barozzi P, Forghieri F, Potenza L. How I treat HHV8/KSHV-related diseases in posttransplant patients. Blood. 2012;120:4150–9.
Smith JM, Rudser K, Gillen D, Kestenbaum B, Seliger S, Weiss N, et al. Risk of lymphoma after renal transplantation varies with time: an analysis of the United States Renal Data System. Transplantation. 2006;81(2):175–80.
Zhang L, Pereira Mestre R, Bihl F, Bühler M, Vannata B, Stathis A. A rare case of classical Hodgkin lymphoma diagnosed 10 years after liver transplant. Case Rep Oncol. 2017;10(3):923–7.
Mucha K, Foroncewicz B, Ziarkiewicz-Wroblewska B, Krawczyk M, Lerut J, Paczek L. Post-transplant lymphoproliferative disorder in view of the new WHO classification: a more rational approach to a protean disease? Nephrology Dialysis Transplantation. 2010;25(7):2089–98.
Hoshida Y, Li T, Dong Z, Tomita Y, Yamauchi A, Hanai J, et al. Lymphoproliferative disorders in renal transplant patients in Japan. Int J Cancer. 2001;91:869–75.
Herreman A, Dierickx D, Morscio J, Camps J, Bittoun E, Verhoef G, et al. Clinicopathological characteristics of posttransplant lymphoproliferative disorders of T-cell origin: single-center series of nine cases and meta-analysis of 147 reported cases. Leuk Lymphoma. 2013;54(10):2190–9.
Swerdlow SH, Campo E, Pileri SA. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. https://doi.org/10.1182/blood-2016-01-643569.
Nelson BP, Wolniak KL, Evens A, Chenn A, Maddalozzo J, Proytcheva M. Early posttransplant lymphoproliferative disease: clinicopathologic features and correlation with mTOR signaling pathway activation. Am J Clin Pathol. 2012;138(4):568–78.
Draoua HY, Tsao L, Mancini DM, Addonizio LJ, Bhagat G, Alobeid B. T-cell posttransplantation lymphoproliferative disorders after cardiac transplantation: a single institutional experience. Br J Haematol. 2004;127:429–32.
Caillard S, Lelong C, Pessione F, Moulin B, French PTLD Working Group. Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French registry. Am J Transplant. 2006;6:2735–42.
Hanson MN, Morrison VA, Peterson BA, Stieglbauer KT, Kubic VL, McCormick S, et al. Posttransplant T-cell lymphoproliferative disorders—an aggressive, late complication of solid-organ transplantation. Blood. 1996;88:3626–33.
Montanari F, Bhagat G, Clark-Garvey S, Seshan V, Zain J, Diefenbach C, et al. Monomorphic T-cell post-transplant lymphoproliferative disorders exhibit markedly inferior outcomes compared to monomorphic B-cell post-transplant lymphoproliferative disorders. Leuk Lymphoma. 2010;51(9):1761–4. https://doi.org/10.3109/10428194.2010.500436.
Krishnamurthy S, Hassan A, Frater JL, Kreisel FH, Paessler ME. Pathologic and clinical features of Hodgkin lymphoma—like posttransplant lymphoproliferative disease. Int J Surg Pathol. 2010;18(4):278–85.
Taoka K, Nannya Y, Yamamoto G, Sakatani T, Ota S, Fukayama M, et al. Progressive transition of Epstein-Barr virus associated lymphoproliferative disease subtypes with the development of lung cancer. Am J Hematol. 2009;84(9):600–3.
Vakiani E, Basso K, Klein U, Mansukhani MM, Narayan G, Smith PM, et al. Genetic and phenotypic analysis of B-cell post-transplant lymphoproliferative disorders provides insights into disease biology. Hematol Oncol. 2008;26(4):199–211.
Reshef R, Morgans AK, Pfanzelter NR, et al. EBV-negative post-transplant lymphoproliferative disorder (PTLD): a retrospective case-control study of clinical and pathological characteristics, response to treatment and survival. Blood. 2008;112:2823.
Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13(2):196–206. https://doi.org/10.1016/S1470-2045(11)70300-X.
Morscio J, Dierickx D, Ferreiro JF, Herreman A, van Loo P, Bittoun E, et al. Gene expression profiling reveals clear differences between EBV-positive and EBV-negative posttransplant lymphoproliferative disorders. Am J Transplant. 2013;13(5):1305–16.
Ferreiro JF, Morscio J, Dierickx D, et al. EBV-positive and EBV-negative Posttransplant diffuse large B cell lymphomas have distinct genomic and transcriptomic features. Am J Transplant. 2016;16(2):414–25. https://doi.org/10.1111/ajt.13558.
Reshef R, Vardhanabhuti S, Luskin MR, Heitjan DF, Hadjiliadis D, Goral S, et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder. Am J Transplant. 2011;11(2):336–47.
Kempf C, Tinguely M, Rushing EJ. Posttransplant lymphoproliferative disorder of the central nervous system. Pathobiology. 2013;80(6):310–8. https://doi.org/10.1159/000347225.
Evens AM, Choquet S, Kroll-Desrosiers AR, Jagadeesh D, Smith SM, Morschhauser F, et al. Primary CNS posttransplant lymphoproliferative disease (PTLD): an international report of 84 cases in the modern era. Am J Transplant. 2013;13(6):1512–22. https://doi.org/10.1111/ajt.12211.
Penn I, Porat G. Central nervous system lymphomas in organ allograft recipients. Transplantation. 1995;59(2):240–4.
Starzl TE, Porter KA, Iwatsuki S, Rosenthal JT, Shaw BW Jr, Atchison RW, et al. Reversibility of lymphomas and lymphoproliferative lesions develo** under cyclosporin-steroid therapy. Lancet. 1984;323:583–7.
LaCasce AS. Post-transplant lymphoproliferative disorders. Oncologist. 2006;11(6):674–80.
Tsai DE, Hardy CL, Tomaszewski JE, Kotloff RM, Oltoff KM, Somer BG, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71:1076–88.
Zimmermann H, Trappe RU. EBV and posttransplantation lymphoproliferative disease: what to do? Hematol Am Soc Hematol Educ Program. 2013;2013:95–102. https://doi.org/10.1182/asheducation-2013.1.95.
Fischer A, Blanche S, Le Bidois J, et al. Anti-B-cell monoclonal antibodies in the treatment of severe B-cell lymphoproliferative syndrome following bone marrow and organ transplantation. N Engl J Med. 1991;324(21):1451–6.
Choquet S, Leblond V, Herbrecht R, Socié G, Stoppa AM, Vandenberghe P, et al. Efficacy and safety of rituximab in B-cell post-transplant lymphoproliferative disorders: results of a prospective multicentre phase II study. Blood. 2006;107:3053–7.
González-Barca E, Domingo-Domenech E, Capote FJ, Gómez-Codina J, Salar A, Bailen A, et al. Prospective phase II trial of extended treatment with rituximab in patients with B-cell post-transplant lymphoproliferative disease. Haematologica. 2007;92(11):1489–94.
Choquet S, Varnous S, Deback C, Golmard JL, Leblond V. Adapted treatment of Epstein-Barr virus infection to prevent posttransplant lymphoproliferative disorder after heart transplantation. Am J Transplant. 2014;14(4):857–66. https://doi.org/10.1111/ajt.12640.
Choquet S, Oertel S, LeBlond V, Riess H, Varoqueaux N, Dörken B, et al. Rituximab in the management of post-transplantation lymphoproliferative disorder after solid organ transplantation: proceed with caution. Ann Hematol. 2007;86(8):599–607.
• Trappe RU, Dierickx D, Zimmermann H, et al. Response to rituximab induction is a predictive marker in B-cell post-transplant lymphoproliferative disorder and allows successful stratification into rituximab or R-CHOP consolidation in an international, prospective, multicenter phase II trial. J Clin Oncol. 2017;35(5):536–43. https://doi.org/10.1200/JCO.2016.69.3564.This 2017 study is one of the few phase II trials of therapy in PTLD which studies a novel stratification schema of PTLD patients to rituximab or R-CHOP therapy based on their initial response to single-agent rituximab.
Choquet S, Trappe R, Leblond V, Jager U, Davi F, Oertel S. CHOP-21 for the treatment of post-transplant lymphoproliferative disorders (PTLD) following solid organ transplantation. Haematologica. 2007;92(2):273–4.
Jagadeesh D, Woda BA, Draper J, Evens AM. Post transplant lymphoproliferative disorders: risk, classification, and therapeutic recommendations. Curr Treat Options in Oncol. 2012;13(1):122–36.
DeStefano CB, Malkovska V, Rafei H, et al. DA-EPOCH-R for post-transplant lymphoproliferative disorders. Eur J Haematol. 2017;99(3):283–5. https://doi.org/10.1111/ejh.12904.
Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–31. https://doi.org/10.1182/blood-2006-12-063008.
Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35. https://doi.org/10.1182/blood-2009-08-239186.
Li JX, Hosoba S, Wayne HAC, et al. Classical Hodgkin lymphoma presenting as a post-transplant lymphoproliferative disorder after organ allograft transplantation. Transl Biomed. 2016;7(2)
Connors JM, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378:331–44. https://doi.org/10.1056/NEJMoa1708984.
Picarsic J, Jaffe R, Mazariegos G, Webber SA, Ellis D, Green MD, et al. Post-transplant Burkitt lymphoma is a more aggressive and distinct form of post-transplant lymphoproliferative disorder. Cancer. 2011;117(19):4540–50.
Zimmermann H, Reinke P, Neuhaus R, Lehmkuhl H, Oertel S, Atta J, et al. Burkitt post-transplantation lymphoma in adult solid organ transplant recipients. Cancer. 2012;118(19):4715–24.
Oertel SH, Papp-Váry M, Anagnostopoulos I, et al. Salvage chemotherapy for refractory or relapsed post-transplant lymphoproliferative disorder in patients after solid organ transplantation with a combination of carboplatin and etoposide. Br J Haematol. 2003;123(5):830–5.
Gibson SE, Swerdlow SH, Craig FE, Surti U, Cook JR, Nalesnik MA, et al. EBV-positive extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in the posttransplant setting: a distinct type of posttransplant lymphoproliferative disorder? Am J Surg Pathol. 2011;35(6):807–15.
Trappe R, Zimmermann H, Fink S, Reinke P, Dreyling M, Pascher A, et al. Plasmacytoma-like post-transplant lymphoproliferative disorder, a rare subtype of monomorphic B-cell post-transplant lymphoproliferation, is associated with a favorable outcome in localized as well as in advanced disease—a prospective analysis of 8 cases. Haematologica. 2011;96(7):1067–71.
Zimmermann H, Oschlies I, Fink S, Pott C, Neumayer HH, Lehmkuhl H, et al. Plasmablastic posttransplant lymphoma: cytogenetic aberrations and lack of Epstein-Barr virus association linked with poor outcome in the prospective german posttransplant lymphoproliferative disorder registry. Transplantation. 2012;93(5):543–50.
Cai Q, Chen K, Young KH. Epstein–Barr virus-positive T/NK-cell lymphoproliferative disorders. Exp Mol Med. 2015;47(1):e133. https://doi.org/10.1038/emm.2014.105.
Mihara A, Chen CK, Yokoyama K, Ueda T, Tsukada Y, Awaya N, et al. Successful treatment with L-asparaginase-based combination chemotherapy for refractory T-cell post-transplant lymphoproliferative disorder. Rinsho Ketsueki. 2007;48(4):305–9.
Kawa K. Diagnosis and treatment of Epstein-Barr virus-associated natural killer cell lymphoproliferative disease. Int J Hematol. 2003;78(1):24–31.
Tsai DE, Aqui NA, Vogl DT, Bloom RD, Schuster SJ, Nasta SD, et al. Successful treatment of T-cell post-transplant lymphoproliferative disorder with the retinoid analog bexarotene. Am J Transplant. 2005;5(8):2070–3.
Lewis DJ, Huang S, Duvic M. Oral bexarotene for post-transplant cutaneous T-cell lymphoma. Dermatol Ther. 2017;30(5) https://doi.org/10.1111/dth.12524.
Portell C, Nand S. Single agent lenalidomide induces a response in refractory T-cell posttransplantation lymphoproliferative disorder. Blood. 2008;111:4416–7.
Choi M, Fink S, Prasad V, et al. T cell PTLD successfully treated with single-agent brentuximab vedotin first-line therapy. Transplantation. 2016;100(3):e8–e10.
Cavaliere R, Petroni G, Lopes MB, Schiff D, The International Primary Central Nervous System Lymphoma Collaborative Group. Primary central nervous system post-transplantation lymphoproliferative disorder. Cancer. 2010;116:863–70.
Patrick A, Wee A, Hedderman A, Wilson D, Weiss J, Govani M. High-dose intravenous rituximab for multifocal, monomorphic primary central nervous system posttransplant lymphoproliferative disorder. J Neuro-Oncol. 2011;103(3):739–43. https://doi.org/10.1007/s11060-010-0425-0.
van de Glind G, de Graaf S, Klein C, Cornelissen M, Maecker B, Loeffen J. Intrathecal rituximab treatment for pediatric post-transplant lymphoproliferative disorder of the central nervous system. Pediatr Blood Cancer. 2008;50(4):886–8.
Bonney DK, Htwe EE, Turner A, Kelsey A, Shabani A, Hughes S, et al. Sustained response to intrathecal rituximab in EBV associated post-transplant lymphoproliferative disease confined to the central nervous system following haematopoietic stem cell transplant. Pediatr Blood Cancer. 2012;58:459–61.
Czyzewski K, Styczynski J, Krenska A, Debski R, Zajac-Spychala O, Wachowiak J, et al. Intrathecal therapy with rituximab in central nervous system involvement of post-transplant lymphoproliferative disorder. Leuk Lymphoma. 2013;54:503–6.
Wu M, Sun J, Zhang Y, et al. Intrathecal rituximab for EBV-associated post-transplant lymphoproliferative disorder with central nervous system involvement unresponsive to intravenous rituximab-based treatments: a prospective study. Bone Marrow Transplant. 2016;51(3):456–8. https://doi.org/10.1038/bmt.2015.281.
Keay S, Oldach D, Wiland A, Klassen D, Schweitzer E, Abruzzo LV, et al. Post-transplant lymphoproliferative disorder associated with OKT3 and decreased antiviral prophylaxis in pancreas transplant recipients. Clin Infect Dis. 1998;26:596–600.
Davis CL, Harrison KL, McVicar JP, et al. Antiviral prophylaxis and the Epstein-Barr virus-related post-transplant lymphoproliferative disorder. Clin Transpl. 1995;9:53–9.
Funch DP, Walker AM, Schneider G, Ziyadeh NJ, Pescovitz MD. Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. Am J Transplant. 2005;5:2894–900.
Opelz G, Daniel V, Naujokat C, Fickenscher H, Döhler B. Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hogkin lymphoma: a multicentre retrospective analysis. Lancet Oncol. 2007;8:212–8.
• AlDabbagh MA, Gitman MR, Kumar D, et al. The role of antiviral prophylaxis for the prevention of Epstein-Barr virus-associated posttransplant lymphoproliferative disease in solid organ transplant recipients: a systematic review. Am J Transplant. 2017;17(3):770–81. https://doi.org/10.1111/ajt.14020.This 2017 systematic review and meta-analysis was performed to answer the question if antiviral prophylaxis is effective in prevention of PTLD, which has mixed findings in the literature.
Wagner HJ, Wessel M, Jabs W, et al. Patients at risk for development of posttransplant lymphoproliferative disorder: plasma versus peripheral blood mononuclear cells as material for quantification of Epstein-Barr viral load by using real-time quantitative polymerase chain reaction. Transplantation. 2001;72:1012–9.
Gil L, Styczynski J, Komarnicki M. Strategy of pre-emptive management of Epstein-Barr virus post-transplant lymphoproliferative disorder after stem cell transplantation: results of European transplant centers survey. Contemp Oncol (Pozn). 2012;16(4):338–40.
Styczynski J, Einsele H, Gil L, Ljungman P. Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis. 2009;11(5):383–92.
McCulloch A, Massey D, Sharkey L, et al. Vitamin D deficiency is associated with an increased risk of post-transplant lymphoproliferative disorder following small intestinal and multivisceral transplantation. Transplantation. 2017;101(6S2):S88. https://doi.org/10.1097/01.tp.0000521412.75744.42.330.16.
Rooney CM, Smith CA, Ng CYC, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–55.
Savoldo B, Goss JA, Hammer MM, Zhang L, Lopez T, Gee AP, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs). Blood. 2006;108(9):2942–9.
Comoli P, Labirio M, Basso S, Baldanti F, Grossi P, Furione M, et al. Infusion of autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for prevention of EBV-related lymphoproliferative disorder in solid organ transplant recipients with evidence of active virus replication. Blood. 2002;99(7):2592–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ajay Major declares that he has no conflict of interest.
Manali Kamdar has received compensation from Seattle Genetics for participation in speaker’s bureaus.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Lymphoma
Rights and permissions
About this article
Cite this article
Major, A., Kamdar, M. Management of Non-Diffuse Large B Cell Lymphoma Post-Transplant Lymphoproliferative Disorder. Curr. Treat. Options in Oncol. 19, 33 (2018). https://doi.org/10.1007/s11864-018-0549-6
Published:
DOI: https://doi.org/10.1007/s11864-018-0549-6