Abstract
Background
While biologic drugs have demonstrated efficacy across a range of indications, patient access to these drugs is constrained due to their high cost. Biosimilars provide a means to increase patient access while reducing the financial burden.
Aims
The primary objective was to determine the current usage of biosimilar and reference trastuzumab and rituximab in four Irish hospitals. A secondary objective involved determining barriers to biosimilar usage.
Methods
This project involved a retrospective chart review to analyse the usage of reference and biosimilar versions of trastuzumab and rituximab. Additionally, a prospective cross-sectional study identified barriers to the usage of biosimilars via the distribution of a novel questionnaire to patients, pharmacists, doctors and students.
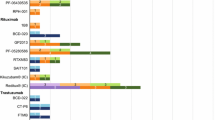
Results
The utilisation of biosimilar intravenous trastuzumab and rituximab ranged from 39 to 100%, and 0 to 89%, respectively. A total of n = 479 questionnaire responses were included. Biosimilar awareness was significantly lower among ‘Doctors and Medical Students’ (45.3%; 95% [CI, 33.8–57.3%]) compared to ‘Pharmacists and Pharmacy Students’ (97.1%; 95% [CI, 94–98.8%; comparison p < 0.001]). A significant majority of healthcare professionals agreed biosimilars should have consistent nomenclature (p < 0.001). A significant majority of patients (87.3%, 95% [CI, 81.3–92%; p < 0.001]) indicated that they would agree to commence using a biosimilar medicine.
Conclusion
Biosimilar versions of trastuzumab and rituximab were in use to a variable extent. There remains a considerable opportunity to further increase the usage to maximise their potential benefits. A series of challenges were identified including reduced awareness among the medical profession and lack of clear nomenclature.



Similar content being viewed by others
References
EMA (2017) Biosimilars in the EU: Information Guide for Healthcare Professionals 2019. Available from: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf
Jacobs I, Singh E, Sewell KL et al (2016) Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Prefer Adherence 10:937–948
Safdar A, Butt MH, Ahmad A, Zaman M (2021) Progress in oncology biosimilars till 2020: scrutinizing comparative studies of biosimilar monoclonal antibodies. J Oncol Pharm Pract 27(5):1195–1204
Ngo D, Chen J (2021) A clinical review of biosimilars approved in oncology. Ann Pharmacother 55(3):362–377
Soares JCS, Cavalcanti IDL, Vasconcelos JLA (2021) Can biosimilar products be interchangeable? Pharmaceutical perspective in the implementation of biosimilars in oncology. J Oncol Pharm Pract 27(6):1491–1502
Duggan B, Smith A, Barry M (2021) Uptake of biosimilars for TNF-alpha inhibitors adalimumab and etanercept following the best-value biological medicine initiative in Ireland. Int J Clin Pharm 43(5):1251–1256
Peeters M, Planchard D, Pegram M et al (2021) Biosimilars in an era of rising oncology treatment options. Future Oncol 17(29):3881–3892
Shelbaya A, Kelton JM, Thompson J et al (2021) Real-world use and acceptance of biosimilar monoclonal antibodies of rituximab in oncology practice in the USA. Future Oncol 17(30):3941–3950
Aschermann LM, Forshay CM, Kennerly-Shah J, Pilz J (2022) The formulary process for biosimilar additions at a comprehensive cancer center. J Oncol Pharm Pract 28(1):185–189
Frank RG, Shahzad M, Kesselheim AS, Feldman W (2022) Biosimilar competition: early learning. Health Econ
Kvien TK, Patel K, Strand V (2022) The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems. Semin Arthritis Rheum 52:151939
Vulto AG, Vanderpuye-Orgle J, van der Graaff M et al (2020) Sustainability of biosimilars in Europe: a Delphi panel consensus with systematic literature review. Pharmaceuticals (Basel] 13(11)
HSE (2019) Guidance for Biological Medicines in Acute Hospitals 2019 [cited 2021 17th October]. Available from: https://www.hse.ie/eng/about/who/acute-hospitals-division/drugs-management-programme/protocols/guidance-for-biological-medicines-in-acute-hospitals.pdf
Cohen H, Beydoun D, Chien D et al (2017) Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther 33(12):2160–2172
van Overbeeke E, De Beleyr B, de Hoon J et al (2017) Perception of originator biologics and biosimilars: a survey among Belgian rheumatoid arthritis patients and rheumatologists. BioDrugs 31(5):447–459
Jang M, Simoens S, Kwon T (2021) Budget impact analysis of the introduction of rituximab and trastuzumab intravenous biosimilars to EU-5 markets. BioDrugs 35(1):89–101
EMA (2021) Trastuzumab, Rituximab 2021 [cited October 17th 2021]. Available from: ema.europa.eu
Lee SM, Jung JH, Suh D et al (2019) Budget impact of switching to biosimilar trastuzumab (CT-P6] for the treatment of breast cancer and gastric cancer in 28 European countries. BioDrugs 33(4):423–436
Leonard E, Wascovich M, Oskouei S et al (2019) Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm 25(1):102–112
Park JH, Yeo JH, Kim YS et al (2022) Efficacy and safety of trastuzumab biosimilar (CT-P6] compared with reference trastuzumab in patients with HER2-positive advanced gastric cancer: a retrospective analysis. Am J Clin Oncol 45(2):61–65
Kurki P, Barry S, Bourges I et al (2021) Safety, immunogenicity and interchangeability of biosimilar monoclonal antibodies and fusion proteins: a regulatory perspective. Drugs 81(16):1881–1896
HPRA (2022) Generic and Interchangeable Medicines 2022. Available from: www.hpra.ie
Candelaria M, González DE, Delamain MT et al (2019) Rituximab biosimilar RTXM83 versus reference rituximab in combination with CHOP as first-line treatment for diffuse large B-cell lymphoma: a randomized, double-blind study. Leuk Lymphoma 60(14):3375–3385. https://doi.org/10.1080/10428194.2019.1633632. Epub 2019 Jul 4. PMID: 31272251
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr Richard Bambury is a co-founder of ‘Portable Medical Technology’. Prof Seamus O’Reilly carries out consulting for Astra Zeneca, receives travel expenses from Roche and Nordic and has meeting registration costs covered by Novartis. Other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Coakley, K.E., Bambury, R.M., McGuinness, E. et al. An evaluation of the utilisation of biosimilar monoclonal antibody drugs in Ireland and barriers to their usage. Ir J Med Sci 193, 1191–1199 (2024). https://doi.org/10.1007/s11845-023-03587-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-023-03587-0