Abstract
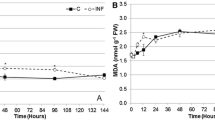
A study was conducted to demonstrate the induced defence responses of chilli plants against herbivory by different larval instars of the tobacco cutworm, Spodoptera litura (F) at different intervals of time (0, 24, 48 and 72 h) after feeding. The rate of various antioxidative enzymes such as peroxidase, catalase (CAT), superoxide and polyphenol oxidases generated in chilli, Capsicum annuum (L) plant leaves due to damage caused by different larval stages of S. litura, and the primary and secondary metabolite contents were quantified. Also, lipid peroxidation content in plant leaves was measured by a malondialdehyde method, while the photosynthetic pigment concentrations were estimated spectrophotometrically. Early instar larval feeding caused an enhanced production of reactive oxygen species (ROS) compared with the older instars. Not much variation occurred in the primary metabolite content of plants fed upon by herbivores, and normal chilly plants. However, a considerable increase in the activities of CAT and superoxide dismutase was recorded after 24 h of insect feeding. Hydrogen peroxide accumulation was higher in plants fed by 2nd instar larvae when estimated after 0 h (immediately after 2 h of feeding was completed), whereas no hydrogen peroxide accumulation was observed due to the feeding by 5th instar larvae using the 3,3′-diaminobenzidine (DAB) staining method. Nitro blue tetrazolium staining for the location of superoxide ions revealed the immediate accumulation of superoxide ions at the damaged site due to the feeding by all the tested instar larvae of S. litura. Among the different larval stages tested, 2nd and 3rd instar feeding led to more superoxide radical accumulation as an indication of ROS generated as a counteraction to herbivory.




Similar content being viewed by others
References
Aebi H (1984) Methods in enzymology. In: Packer L (ed.) Academic press, Orlando, pp. 121–126
Argandoña VH, Chaman M, Cardemil L, Muñoz O, Zúñiga GE, Corcuera LJ (2001) Ethylene production and peroxidase activity in aphid-infested barley. J Chem Ecol. doi:10.1023/A:1005615932694
Arnold T, Appel H, Patel V, Stocum E, Kavalier A, Schultz J (2004) Carbohydrate translocation determines the phenolic content of Populus foliage: a test of the sink–source model of plant defense. New Phytol 164:157–164
Arnon DJ (1949) Copper enzymes in isolated chloroplast. Polyphenol oxidase in Beta Vulgaris. Plant Physiol 24:1–15
Barakat A, Bagniewska-Zadworna A, Frost CJ, Carlson JE (2010) Phylogeny and expression profiling of CAD and CAD-like genes in hybrid Populus (P. deltoides × P nigra): evidence from herbivore damage for subfunctionalization and functional divergence. BMC Plant Biol. doi:10.1186/1471-2229-10-100
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bhonwong A, Stout MJ, Attajarusit J, Tantasawat P (2009) Defensive role of tomato polyphenol oxidases against cotton bollworm (Helicoverpa armigera) and beet armyworm (Spodoptera exigua). J Chem Ecol. doi:10.1007/s10886-008-9571-7
Bi JL, Felton GW (1995) Foliar oxidative stress and insect herbivory—primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J Chem Ecol 21:1511–1530
Bolton MD (2009) Primary metabolism and plant defense—fuel for the fire. Mol Plant Microbe Interact 22(5):487–497
Constabel CP, Bergey DR, Ryan CA (1995) Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proc Natl Acad Sci USA 92:407–411
Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24(1):275–287
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fanizza G, Ricciardi L, Bagnulo C (1991) Leaf greenness measurements to evaluate water stressed genotypes in Vitis vinifera. Euphytica 55:27–31
Fukao T, Bailey-Serres J (2004) Plant responses to hypoxia-is survival a balancing act. Trends Plant Sci 9:449–456
Goggin FL, Avila CA, Lorence A (2010) Vitamin C content in plants is modified by insects and influences susceptibility to herbivory. BioEssays 32:777–790
Halliwell B, Gutteridge JMC (1989) Lipid peroxidation: a radical chain reaction. Free radicals in biology and medicine. Clarendon Press, Oxford, pp 139–187
Han C, Liu Q, Yang Y (2009) Short-term effects of experimental warming and enhanced ultraviolet-B radiation on photosynthesis and antioxidant defense of Picea asperata seedlings. Plant Growth Regul 58:153–162
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Part I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hori K (1973) Studies on the feeding habits of Lygus disponsi Linnavuori (Hemiptera: Miridae) and the injury to its host plant. III: Phenolic compounds, acid phosphatase and oxidative enzymes in the injured tissue of sugar beet leaf. Appl Entomol Zool 8(2):103–112
Jiang Y, Huang B (2001) Drought and heat injuiry to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci 41:436–442
Johnson MTJ, Smith SD, Rausher MD (2009) Plant sex and the evolution of plant defenses against herbivores. Proc Natl Acad Sci USA 106:18079–18084
Kanchiswamy CN, Takahashi H, Quadro S, Maffei ME, Bossi S, Bertea C et al (2010) Regulation of arabidopsis defense responses against Spodoptera littoralis by CPK-mediated calcium signaling. BMC Plant Biol. doi:10.1186/1471-2229-10-97
Kar M, Mishra D (1976) Catalase, peroxidase and polyphenol oxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328
Kumar H, Sharma S (2014) Determination of chlorophyll and carotenoid loss in Dalbergia sissoo caused by Aonidiella orientalis (Newstead) [Homoptera: Coccoidea: Diaspididae]. J Entomol Zool Stud 2(1):104–106
Laloi C, Apel K, Danon A (2004) Reactive oxygen signaling: the latest news. Curr Opin Plant Biol 7:323–328
Lowry OH, Rosenbrough NJ, Farrand AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Machado RAR, Ferrieri AP, Robert CAM, Glauser G, Kallenbach M, Baldwin IT, Erb M (2013) Leaf-herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytol 200:1234–1246
Machado RAR, Arce CCM, Ferrieri AP, Baldwin IT, Erb M (2015) Jasmonate-dependent depletion of soluble sugars compromises plantresistance to Manduca sexta. New Phytol 207:91–105
Maffei ME, Mithöfer A, Boland W (2007) Insects feeding on plants: rapid signals and responses preceding the induction of phytochemical release. Phytochem. doi:10.1016/j.phytochem.2007.07.016
Mehdy MC, Sharma YK, Kanagasabapathi S, Bays NW (1996) The role of activated oxygen species in plant disease resistance. Physiol Plant 98(2):365–374
Mishra SA, Jha B, Dubey RS (2011) Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 48(3):565–577
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plant. Trends Plant Sci 9(10):490–498
Moore S, Stein WH (1954) A modified ninhydrin reagent for the photometric determination of aminoacids and related compounds. J Biol Chem 211:907–913
Noctor G, Foyer CH (1998) Ascorbate and glutathione: kee** active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Orozco-cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96:6553–6557
Rani PU, Jyothsna Y (2010) Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physiol Plant 32:695–701
Sairam RK, Shukla DS, Saxena DC (1997) Stress induced injuiry and antioxidant enzymes in relation to drought tolerance in wheat genotypes. Biol Plantarum 40:357–364
Sambangi P, Usha Rani P (2013) Induction of phenolic acids and metals in Arachis hypogaea L. plants due to feeding of three lepidopteran pests. Arthropod Plant Interact 7:517–525
Scholes JD, Lee PJ, Horton P, Lewis DH (1994) Invertase: understanding changes in the photosynthetic and carbohydrate-metabolism of barley leaves infected with powdery mildew. New Phytol 126(2):213–222
Schwachtje J, Baldwin IT (2008) Why does herbivore attack reconfigure primary metabolism? Plant Physiol 146:845–851
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46:209–221
Sharma P, Jha AB, Dubey RS (2010) Oxidative stress and antioxidative defense system in plants growing under abiotic stresses. In: Pessarakli M (ed) Handbook of Plant and Crop Stress, 3rd edn. CRC Press, Boca Raton, FL, pp 89–138
Takabayashi J, Takahashi S, Dicke M, Posthumus MA (1995) Developmental stage of herbivore Pseudaletia separata affects production of herbivore-induced synomone by corn plants. J Chem Ecol 21:273–287
Tanou G, Molassiotis A, Diamantidis G (2009) Induction of reactive oxygen species and necrotic death-like destruction in strawberry leaves by salinity. Environ Exp Bot 65(2–3):270–281
Thaler JS, Stout MJ, Karban R, Duffey SS (1996) Exogenous jasmonates simulate insect wounding in tomato plants, Lycopersicon esculentum, in the laboratory and field. J Chem Ecol 22:1767–1781
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11(6):1187–1194
Torres MA (2010) ROS in biotic interactions. Physiol Plant. doi:10.1111/j.1399-3054.2009.01326.x
Usha Rani P, Ravibabu MV (2011) Allelochemicals in castor (Ricinus communis) plants and their impact on pest larval feeding as anti-herbivore defensive. Allelopathy J 27:263–276
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
War AR, Paulraj MG, War MY, Ignacimuthu S (2011a) Jasmonic acid-mediated induced resistance in groundnut (Arachis hypogaea L.) against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J Plant Growth Regul 30:512–523
War AR, Paulraj MG, War MY, Ignacimuthu S (2011b) Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L.). Plant Signal Behav 6:1787–1792
Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Kangasjärvi J, Sandermann H, Langebartels C (2002) Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant, Cell Environ 25:717–726
Wojtaszek P (1997) Oxidation burst: an early plant response to pathogen infection. Biochem J 322:681–692
Yasur J, Usha Rani P (2009) Oxidative response of castor (Ricinus communis L.) and rice (Oryza sativa L.) plants due to herbivory. Insect Pest Manag Environ Safety 4(1):69–75
Acknowledgements
One of the authors (MV) is grateful to the Department of Science and Technology, INSPIRE, New Delhi for a research grant, and to the Director, CSIR-Indian Institute of Chemical Technology for the facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Rupesh Kariyat.
Rights and permissions
About this article
Cite this article
Vijaya, M., Rani, P.U. Defensive responses in Capsicum annuum (L) plants, induced due to the feeding by different larval instars of Spodoptera litura (F). Arthropod-Plant Interactions 11, 193–202 (2017). https://doi.org/10.1007/s11829-016-9479-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-016-9479-z