Abstract
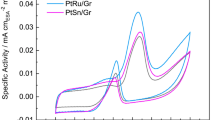
Electrochemically exfoliated graphene (EEG) is a kind of high-quality graphene with few oxygen-containing functional groups and defects on the surface, and thereby is more suitable as catalyst support than other carbon materials such as extensively used reduced graphene oxide (rGO). However, it is difficult to grow functional materials on EEG due to its inert surface. In this work, ultra-small Pt nanocrystals (∼2.6 nm) are successfully formed on EEG and show better electrocatalytic activity towards methanol oxidation than Pt catalysts on rGO. The outstanding catalytic properties of Pt catalysts on EEG can be attributed to the fast electron transfer through EEG and high quality of Pt catalysts such as small grain size, high dispersibility and low oxidation ratio. In addition, SnO2 nanocrystals are controllably generated around Pt catalysts on EEG to raise the poison tolerance of Pt catalysts through using glycine as a linker. Owing to its outstanding properties such as high electrical conductivity and mechanical strength, EEG is expected to be widely used as a novel support for catalysts.
摘要
电化学剥离石墨烯是一种高质量的石墨烯, 其表面几乎没有含氧官能团和缺陷, 因此比其他碳 材料(例如广泛使用的还原氧化石墨烯)更适合作为催化剂载体。然而, 由于石墨烯的惰性表面, 其功 能材料很难在电化学剥离的石墨烯上生长。在这项工作中, 超小的Pt 纳米晶(∼2.6 nm)成功地生长在 电化学剥离的石墨烯表面, 对甲醇氧化的电催化活性优于还原氧化石墨烯负载的Pt 催化剂。电化学 剥离石墨烯负载的铂催化剂具有优异的电催化性能, 这主要归功于电化学剥离石墨烯与铂之间的快速 电子转移, 同时, 电化学剥离石墨烯负载的铂催化剂具有颗粒尺寸小、分散性高、氧化态Pt 含量低 等优点。此外, 利用甘氨酸作为连接剂, 在电化学剥离石墨烯上可以控制铂催化剂周围生成SnO2 纳 米晶, 进一步增**铂催化剂的抗中毒能力。电化学剥离石墨烯具有良好的导电性和机械**度, 有望作 为新型催化剂载体得到广泛应用。
Similar content being viewed by others
References
LIU Min-min, ZHANG Rui-zhong, CHEN Wei. Graphene-supported nanoelectrocatalysts for fuel cells: Synthesis, properties, and applications [J]. Chem Rev, 2014, 114: 5117–5160. DOI: https://doi.org/10.1021/cr400523y.
LONG Gui-fa, LI **ao-hua, WAN Kai, LIANG Zhen-xing, PIAO **-hua, TSIAKARAS P. Pt/CN-doped electrocatalysts: Superior electrocatalytic activity for methanol oxidation reaction and mechanistic insight into interfacial enhancement [J]. Appl Catal B: Environ, 2017, 203: 541–548. DOI: https://doi.org/10.1016/j.apcatb.2016.10.055.
KIM D H, SHIN D Y, LEE Y G, AN G H, HAN J H, AHN H J, CHOI B J. Effects of SnO2 layer coated on carbon nanofiber for the methanol oxidation reaction [J]. Ceram Int, 2018, 44: 19554–19559. DOI: https://doi.org/10.1016/j.ceramint.2018.07.199.
WANG Li-**, TIAN **g, LI **g-sha, ZENG **an-guang, PENG Zhi-guang, HUANG **ao-bing, TANG You-gen, WANG Hai-yan. Red-blood-cell-like nitrogen-doped porous carbon as an efficient metal-free catalyst for oxygen reduction reaction [J]. J Cent South Univ, 2019, 26: 1459–1468. DOI: https://doi.org/10.1007/s11771-019-4102-y.
CHEN Tsan-yao, HUANG Po-chun, LIAO Yen-fa, LIU Yu-ting, TEH Tsung-kuang, LIN Tsang-lang. Shell thickness effects on reconfiguration of NiOcore-Ptshell anodic catalysts in a high current density direct methanol fuel cell [J]. RSC Adv, 2016, 6: 72607–72615. DOI: https://doi.org/10.1039/c6ra17013g.
XIA Zhang-xun, XU **n-long, ZHANG **ao-ming, LI Huan-qiao, WANG Su-li, SUN Gong-quan. Anodic engineering towards high-performance direct methanol fuel cells with non-precious-metal cathode catalysts [J]. J Mater Chem A, 2020, 8: 1113–1119. DOI: https://doi.org/10.1039/c9ta11440h.
NAN Li-rui, YUE Wen-bo, JIANG Yang. Fabrication of graphene-porous carbon-Pt nanocomposites with high electrocatalytic activity and durability for methanol oxidation [J]. J Mater Chem A, 2015, 3: 22170–22175. DOI: https://doi.org/10.1039/c5ta06854a.
ZHANG Yang-**, GAO Fei, SONG **-**, WANG **, SHIRAISHI Y, DU Yu-kou. Glycine-assisted fabrication of N-Doped graphene-supported uniform multipetal PtAg nanoflowers for enhanced ethanol and ethylene glycol oxidation [J]. ACS Sustainable Chem Eng, 2019, 7: 3176–3184. DOI: https://doi.org/10.1021/acssuschemeng.8b05020.
QIN Yong, CHAO Lei, YUAN Jie, LIU Yang, CHU Fu-qiang, KONG Yong, TAO Yong-xie, LIU Mei-lin. Ultrafine Pt nanoparticle-decorated robust 3D N-doped porous graphene as an enhanced electrocatalyst for methanol oxidation [J]. Chem Commun, 2016, 52: 382–385. DOI: https://doi.org/10.1039/c5cc07482g.
HE Yuan, LIU Yun-guo. Direct fabrication of highly porous graphene/TiO2 composite nanofibers by electrospinning for photocatalytic application [J]. J Cent South Univ, 2018, 25: 2182–2189. DOI: https://doi.org/10.1007/s11771-018-3906-5.
ZHU Cheng-zhou, WANG **, WANG Li, HAN Lei, DONG Shao-jun. Facile synthesis of two-dimensional graphene/SnO2/Pt ternary hybrid nanomaterials and their catalytic properties [J]. Nanoscale, 2011, 3: 4376–4382. DOI: https://doi.org/10.1039/c1nr10634a.
GAO Li-na, YUE Wen-bo, TAO Shan-shan, FAN Lou-zhen. Novel strategy for preparation of Graphene-Pd, Pt composite, and its enhanced electrocatalytic activity for alcohol oxidation [J]. Langmuir, 2013, 29: 957–964. DOI: https://doi.org/10.1021/la303663x.
QU Yun-teng, GAO Yun-zhi, WANG Long, RAO Jian-cun, YIN Ge-**. Mild synthesis of Pt/SnO2/graphene nanocomposites with remarkably enhanced ethanol electro-oxidation activity and durability [J]. Chem Eur J, 2016, 22: 193–198. DOI: https://doi.org/10.1002/chem.201503867.
WEI Wei, WANG Gang, YANG Sheng, FENG **n-liang, MULLEN K. Efficient coupling of nanoparticles to electrochemically exfoliated graphene [J]. J Am Chem Soc, 2015, 137: 5576–5581. DOI: https://doi.org/10.1021/jacs.5b02284.
CHEN Chia-hsuan, YANG Shiou-wen, CHUANG Min-chiang, WOON Wei-yen, SU Ching-yuan. Towards the continuous production of high crystallinity graphene via electrochemical exfoliation with molecular in situ encapsulation [J]. Nanoscale, 2015, 7: 15362–15373. DOI: https://doi.org/10.1039/c5nr03669k.
YANG Sheng, BRULLER S, WU Zhong-shuai, LIU Zhao-yang, PARVEZ K, DONG Ren-hao, RICHARD F, SAMORI P, FENG **n-liang, MULLEN K. Organic radical-assisted electrochemical exfoliation for the scalable production of high-quality graphene [J]. J Am Chem Soc, 2015, 137: 13927–13932. DOI: https://doi.org/10.1021/jacs.5b09000.
YANG Sheng, LOHE M R, MULLEN K, FENG **n-liang. New-generation graphene from electrochemical approaches: Production and applications [J]. Adv Mater, 2016, 28: 6213–6221. DOI: https://doi.org/10.1002/adma.201505326.
XU Ze-xuan, YUE Wen-bo, LIN Rong, CHIANG Chang-yang, ZHOU Wu-zong. Direct growth of SnO2 nanocrystallites on electrochemically exfoliated graphene for lithium storage [J]. J Energy Storage, 2019, 21: 647–656. DOI: https://doi.org/10.1016/j.est.2019.01.001.
XU Ze-xuan, ZHANG **, CHEN Jia-lu, YUE Wen-bo, ZHOU Wu-zong. Growth and growth mechanism of oxide nanocrystals on electrochemically exfoliated graphene for lithium storage [J]. Energy Storage Mater, 2019, 18: 174–181. DOI: https://doi.org/10.1016/j.ensm.2018.08.023.
NAN Li-rui, FAN Ze-tan, YUE Wen-bo, DONG Qiao, ZHU Li-sha, YANG Liu, FAN Lou-zhen. Graphene-based porous carbon-Pd/SnO2 nanocomposites with enhanced electrocatalytic activity and durability for methanol oxidation [J]. J Mater Chem A, 2016, 4: 8898–8904. DOI: https://doi.org/10.1039/c6ta01416j.
ZHANG Jun, LIU **ang-hong, GUO **an-zhi, WU Shi-hua, WANG Shu-rong. A general approach to fabricate diverse noble-metal (Au, Pt, Ag, Pt/Au)/Fe2O3 hybrid nanomaterials [J]. Chem Eur J, 2010, 16: 8108–9700. DOI: https://doi.org/10.1002/chem.201000096.
YANG Sheng, RICCIARDULLI A G, LIU Shao-hua, DONG Ren-hao, LOHE M R, BECKER A, SQUILLACI M A, SAMORI P, MULLEN K, FENG **n-liang. Ultrafast delamination of graphite into high-quality graphene using alternating currents [J]. Angew Chem Int Ed, 2017, 56: 6669–6675. DOI: https://doi.org/10.1002/anie.201702076.
TURGUT H, TIAN Z R, YU Feng-jiao, ZHOU Wu-zong. Multivalent cation cross-linking suppresses highly energetic graphene oxide’s flammability [J]. J Phys Chem C, 2017, 121: 5829–5835. DOI: https://doi.org/10.1021/acs.jpcc.6b13043.
ZHAO Peng, YUE Wen-bo, YUAN Xu, BAO Hua-ying. Exceptional lithium anodic performance of Pd-doped graphene-based SnO2 nanocomposite [J]. Electrochim Acta, 2017, 225: 322–329. DOI: https://doi.org/10.1016/j.electacta.2016.12.124.
LI Zhong-shui, XU Shu-hong, XIE Yi-xie, WANG Yan-lin, LIN Shen. Promotional effects of trace Bi on its highly catalytic activity for methanol oxidation of hollow Pt/graphene catalyst [J]. Electrochim Acta, 2018, 264: 53–60. DOI: https://doi.org/10.1016/j.electacta.2018.01.096.
CHEN Ke, ZHANG Fei, SUN **g-yu, LI Zhen-zhu, ZHANG Li, BACHMATIUK A, ZOU Zhi-yu, CHEN Zhao-long, ZHANG Li-ya, RUMMELI M H, LIU Zhong-fan. Growth of defect-engineered graphene on manganese oxides for Li-ion storage [J]. Energy Storage Mater, 2018, 12: 110–118. DOI: https://doi.org/10.1016/j.ensm.2017.12.001.
PARVEZ K, WU Zhong-shuai, LI Rong-**, LIU **an-jie, GRAF R, FENG **n-liang, MULLEN K. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts [J]. J Am Chem Soc, 2014, 136: 6083–6091. DOI: https://doi.org/10.1021/ja5017156.
CHU Yuan-yuan, ZHANG Ning, YANG **g-**g, WANG Hai-tao, DAI Zhao, WANG Liang, GAO Jun, TAN **ao-yao. Designed synthesis of thin CeO2 nanowires-supported Pt electrocatalysts with pore-interconnected structure and its high catalytic activity for methanol oxidation [J]. J Mater Sci, 2018, 53: 2087–2101. DOI: https://doi.org/10.1007/s10853-017-1636-y.
FENG Yuan-yuan, BI Li-xiao, LIU Zeng-hua, KONG De-sheng, YU Zhang-yu. Significantly enhanced electrocatalytic activity for methanol electro-oxidation on Ag oxide-promoted PtAg/C catalysts in alkaline electrolyte [J]. J Catal, 2012, 290: 18–25. DOI: https://doi.org/10.1016/j.jcat.2012.02.013.
YANG Pei-pei, YUAN **ao-lei, HU Hui-cheng, LIU Yi-lin, ZHENG Hao-wen, YANG Di, CHEN Lei, CAO Mu-han, XU Yong, MIN Yu-lin, LI Yan-guang, ZHANG Qiao. Solvothermal synthesis of alloyed ptni colloidal nanocrystal clusters (CNCs) with enhanced catalytic activity for methanol oxidation [J]. Adv Funct Mater, 2018, 28: 1704774. DOI: https://doi.org/10.1002/adfm.201704774.
QIAN Wen, HAO Rui, ZHOU Jian, EASTMAN M, MANHAT B A, SUN Qiang, GOFORTH A M, JIAO Jun. Exfoliated graphene-supported Pt and Pt-based alloys as electrocatalysts for direct methanol fuel cells [J]. Carbon, 2013, 52: 595–604. DOI: https://doi.org/10.1016/j.carbon.2012.10.031.
NAN Li-rui, YUE Wen-bo. Exceptional electrocatalytic activity and selectivity of platinum@Nitrogen-doped mesoporous carbon nanospheres for alcohol oxidation [J]. ACS Appl Mater Interfaces, 2018, 10: 26213–26221. DOI: https://doi.org/10.1021/acsami.8b06347.
DU De-jian, DU Yi-en, YUE Wen-bo, YANG **ao-**g. Lithium storage performance of {010}-faceted and [111]-faceted anatase TiO2 nanocrystals [J]. J Cent South Univ, 2019, 26: 1530–1539. DOI: https://doi.org/10.1007/s11771-019-4109-4.
WU Shou-liang, LIU Jun, LIANG De-wei, SUN Hong-mei, YE Yi-xing, TIAN Zhen-fei, LIANG Chang-hao. Photo-excited in situ loading of Pt clusters onto rGO immobilized SnO2 with excellent catalytic performance toward methanol oxidation [J]. Nano Energy, 2016, 26: 699–707. DOI: https://doi.org/10.1016/j.nanoen.2016.06.038.
FENG Hong-bin, LIU Yong, LI **g-hong. Highly reduced graphene oxide supported Pt nanocomposites as highly efficient catalysts for methanol oxidation [J]. Chem Commun, 2015, 51: 2418–2420. DOI: https://doi.org/10.1039/c4cc09146a.
WANG Ming-jun, SONG Xue-fen, YANG Qi, HUA Hao, DAI Shu-ge, HU Chen-guo, WEI Da-peng. Pt nanoparticles supported on graphene three-dimensional network structure for effective methanol and ethanol oxidation [J]. J Power Sources, 2015, 273: 624–630. DOI: https://doi.org/10.1016/j.jpowsour.2014.09.117.
ZHAO Jian, YU Hui, LIU Zhen-sheng, JI Min, ZHANG Li-qing, SUN Guang-wei. Supercritical deposition route of preparing Pt/Graphene composites and their catalytic performance toward methanol electrooxidation [J]. J Phys Chem C, 2014, 118: 1182–1190. DOI: https://doi.org/10.1021/jp402620p.
WU Shou-liang, LIU Jun, TIAN Zhen-fei, CAI Yun-yu, YE Yi-xing, YUAN Qing-lin, LIANG Chang-hao. Highly dispersed ultrafine pt nanoparticles on reduced graphene oxide nanosheets: in situ sacrificial template synthesis and superior electrocatalytic performance for methanol oxidation [J]. ACS Appl Mater Interfaces, 2015, 7: 22935–22940. DOI: https://doi.org/10.1021/acsami.5b06153.
ZHOU Yi-ge, CHEN **g-**g, WANG Feng-bin, SHENG Zhen-huan, XIA **ng-hua. A facile approach to the synthesis of highly electroactive Ptnanoparticles on graphene as an anode catalyst for direct methanolfuelcells [J]. Chem Commun, 2010, 46: 5951–5953. DOI: https://doi.org/10.1039/c0cc00394h.
LIANG Qing-sheng, ZHANG Li, CAI Mao-lin, LI Yong, JIANG Kun, ZHANG **n, SHEN Pei-kang. Preparation and charaterization of Pt/functionalized graphene and its electrocatalysis for methanol oxidation [J]. Electrochim Acta, 2013, 111: 275–283. DOI: https://doi.org/10.1016/j.electacta.2013.07.198.
CHOI Sung-mook, SEO Min-ho, KIM Hyung-ju, KIM Won-bae. Synthesis of surface-functionalized graphene nanosheets with high Pt-loadings and their applications to methanol electrooxidation [J]. Carbon, 2011, 49: 904–909. DOI: https://doi.org/10.1016/j.carbon.2010.10.055.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item
Projects(21573023, 21975030) supported by the National Natural Science Foundation of China
Contributors
YUAN Xu: Investigation, methodology, writing-original draft. ZHANG **: Investigation, validation. YUE Wen-bo: Supervision, conceptualization, writing-reviewing & editing, funding acquisition.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Yuan, X., Yue, Wb. & Zhang, J. Electrochemically exfoliated graphene as high-performance catalyst support to promote electrocatalytic oxidation of methanol on Pt catalysts. J. Cent. South Univ. 27, 2515–2529 (2020). https://doi.org/10.1007/s11771-020-4477-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-020-4477-9