Abstract
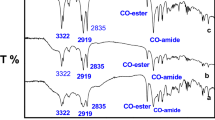
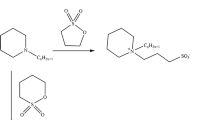
Two series of diquaternary cationic surfactants designated as E9Nm and E11Nm having two different alkyl chains in their chemical structure were synthesized. The chemical structures of these surfactants were confirmed using elemental analysis, FTIR and 1H-NMR spectra. The surface activities of the different surfactants were determined using surface and interfacial tension at 25 °C. The surface parameters including: critical micelle concentration, effectiveness, efficiency, maximum surface excess and minimum surface area were determined. The surface activities of the cationic surfactants were correlated with their chemical structure. The surface activities of the surfactants increased with increasing the hydrophobic chain length. The adsorption and micellization tendencies of the surfactants in solution were determined using the free energies of adsorption and micellization. The synthesized surfactants were evaluated as biocides against bacteria and fungi. Biocidal activity data showed that a gradual increase in the hydrophobic chain length of the surfactant molecules gradually increases the efficiency of these surfactants as biocides.










Similar content being viewed by others
References
Benvegnu T, Plusquellec D, Lemiegre L, Belgacem MN, Gandini A (2008) Monomers, polymers and composites from renewable resources. Elsevier, Amsterdam
El-Sukkary MMA, Shaker NO, Ismail DA, Zaki MF, Ahmed SM (2012) Surface parameters, biodegradability and antimicrobial activity of some amide ether carboxylates surfactants. Egypt J Pet 21:37–43
El-Sukkary MMA, Shaker NO, Ismail DA, Ahmed SM, Awad AI (2012) Preparation and evaluation of some amide ether carboxylate surfactants. Egypt J Pet 21:11–17
Steichen DS, Holmberg K (2001) Handbook of applied surface and colloid chemistry, vol 1. Wiley, New York, pp 309–314
Steichen DS, Gadberry JF, Karsa DR (1998) New products and applications in surface technology. Sheffield Academic Press, Sheffield, p 59
Cross J, Singer EJ (1994) Cationic surfactants. Marcel Dekker, New York, p 3
Huber L, Nitschke L, Holmberg K (2002) Handbook of applied surface and colloid chemistry, vol 1. Wiley, Chichester, pp 509–516
Stjerndahl M, Landberg D, Holmberg K (2003) Novel surfactants, preparation, application and biodegradability. Marcel Dekker, New York, pp 317–321
Tehrani-Bagha AR, Holmberg K (2007) Cleavable surfactants. Curr Opin J Colloid Interface Sci 12:81–84
Rosen MJ (1993) Geminis: a new generation of surfactants. Chem Tech 23:30–33
Menger FM, Littau CA (1993) Gemini surfactants a new class of self-assembling molecule. J Am Chem Soc 115:10083–10090
Zana R (2002) Dimeric gemini surfactants effect of the spacer group on the associate behavior in aqueous solution. J Colloid Interface Sci 248:203–220
Zana R (2002) Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution a review. Adv Colloid Interface Sci 97:205–253
Aisaka T, Oida T, Kawase T (2007) A novel synthesis of succinic acid type Gemini surfactant by the function group interconversion of corynomicolic acid. J Oleo Sci 56:633
Bunton CA, Robinson L, Schaak J, Stam MF (1971) Catalysis of nucleophilic substitution by micelles of dicationic detergents. J Org Chem 36:2346–2350
Negm NA, Badr EA, Aiad IA, Zaki MF, Said MM (2012) Investigation the inhibitory action of novel diquaternary di Schiff bases on the acid dissolution of carbon steel in 1 M hydrochloric acid solution. Corros Sci 65:77–86
EL-Sukkary MMA, Ismail DA, El Rayes SM, Saad MA (2014) Synthesis and evaluation of some derivatives of polysiloxanes. Egypt J Pet 23:361–366
Negm NA, El Farargy AFM, Mohammed DE, Mohamad HN (2012) Environmentally friendly nonionic surfactants derived from tannic acid: synthesis, characterization and surface activity. J Surfactants Deterg 15:433–443
Negm NA (2007) Solubilization, surface active and thermodynamic parameters of gemini amphiphiles bearing nonionic hydrophilic spacer. J Surfactants Deterg 8:71–80
Negm NA, El Farargy AF, Al Sabagh AM, Abdelrahman NR (2011) New Schiff base cationic surfactants: surface and thermodynamic properties and applicability in bacterial growth and metal corrosion prevention. J Surfactants Deterg 14:505–514
Negm NA, Mohamed AA, El-Awady MY (2005) Influence of structure of the cationic polytriethanol ammonium bromide derivatives II. Corrosion inhibition. Egypt J Pet 14:201–210
National Committee for Clinical Laboratory Standards; methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. National Committee for Clinical Laboratory standards, Wayne (1997)
Negm NA, Mohamed AS (2008) Synthesis, characterization and biological activity of sugar-based gemini cationic amphiphiles. J Surfactants Deterg 11:215–221
Negm NA, Said MM, Morsy SMI (2010) Pyrazole derived cationic surfactants and their tin and copper complexes: synthesis, surface activity, antibacterial and antifungal efficacy. J Surfactants Deterg 13:521–528
Wegrzynska J, Chlebicki J (2006) Surface-active antielectrostatic properties of multiple quaternary ammonium salts. J Surfactants Deterg 9:221
Chlebicki J, Wegrzynska M, Oswiecimska J, Maliszewska I (2005) Preparation, surface-active properties, and antimicrobial activities of bis-quaternary ammonium salts from amines and epichlorohydrin. J Surfactants Deterg 8:227
Rosen MJ (2001) Surface and interfacial phenomena, 2nd edn. John Wiley, New York, p 151
El-Sukkary MMA, Ghuiba FM, Sayed GH, Abdou MI, Badr EA, Tawfik SM, Negm NA (2014) Evaluation of some vanillin-modified polyoxyethylene surfactants as additives for water based mud. Egypt J Pet 23:7–14
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Migahed, M.A., Negm, N.A., Shaban, M.M. et al. Synthesis, Characterization, Surface and Biological Activity of Diquaternary Cationic Surfactants Containing Ester Linkage. J Surfact Deterg 19, 119–128 (2016). https://doi.org/10.1007/s11743-015-1749-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1749-8