Abstract
Robotic pyeloplasty has become a natural progression from the development of open, then laparoscopic procedures to treat pediatric patients with ureteropelvic junction obstruction (UPJO). Robotic-assisted pyeloplasty (RALP) is now considered a new gold standard in pediatric MIS. A systematic review of the literature retrieved from PubMed and published in the last 10 years (2012–2022) was performed. This review underlines that in all children except the smallest infants, where the open procedure has benefits in terms of duration of general anesthetic and there are limitations in the size of instruments, robotic pyeloplasty is becoming the preferred procedure to perform in patients with UPJO. Results for the robotic approach are extremely promising, with shorter operative times than laparoscopy and equal success rates, length of stay and complications. In case of redo pyeloplasty, RALP is easier to perform than other open or MIS procedures. By 2009, robotic surgery became the most used modality to treat all UPJO and continues to grow in popularity. Robot-assisted laparoscopic pyeloplasty in children is safe and effective with excellent outcomes, even in redo pyeloplasty or challenging anatomical cases. Moreover, robotic approach shortens the learning curve for junior surgeons, who can readily achieve levels of expertise comparable to senior practitioners. However, there are still concerns regarding the cost associated with this procedure. Further high-quality prospective observational studies and clinical trials, as well as new technologies specific for the pediatric population, are advisable for RALP to reach the level of gold standard.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrinsic or extrinsic compression of the ureteropelvic junction (UPJ) caused, respectively, by fibrosis/stenosis of the proximal ureter or aberrant lower pole vessels is a common issue in pediatric urology. In the last years, prenatal hydronephrosis has been found in up to 4.5% screening ultrasounds, with UPJO being the cause in up to 41% of cases [1]. Despite a large proportion of UPJO-like (isolated hydronephrosis, with or without dilated calyces) cases being benign in nature and spontaneously resolve [1], approximately 25% of prenatally diagnosed UPJO require surgery [2]. This diagnostic change has significantly decreased the age of pyeloplasty. Many attempts have been made to simplify the procedure and minimize complications since its first description by Anderson and Hynes in 1949 [3]. Until now, the gold standard for the treatment of UPJ obstruction is still the Anderson–Hynes dismembered pyeloplasty, traditionally performed with an open flank approach, which has an overall success rate ranging from 90 to 100% [3, 4]. In 1995, the first reported pediatric laparoscopic pyeloplasty (LP) was performed [4]. Some years later, the technique was confirmed as a safe and effective minimally invasive treatment alternative for UPJO [4], but a challenging procedure in terms of intracorporeal suturing and knotting, ergonomics and learning curve [4, 5]. The continued interest in minimally invasive treatment for UPJO has inspired new questions about the optimal approach for treating this disease. However, the general application of LP in children was very limited, until the da Vinci system became available in 2002 and the first robot-assisted laparoscopic pyeloplasty (RALP) series was evaluated [5]. The last 10 years have been characterized by continuous improvements in robotic surgery for the pediatric population and RALP has become the most performed robotic procedure in pediatric urology [6], with reported success rates of 95%–100% [7, 8]. This review aims to discuss important and somewhat controversial aspects of RALP in children, mainly: surgical indications, success rate and complications, redo surgery, challenging cases, cost considerations, and training and learning curve, along with a special highlight on the future of robotic surgery in the treatment of pediatric ureteropelvic junction obstruction in children.
Material and methods
This study aimed to review the international literature of the past 10 years, 2012–2022, focused on robotic-assisted pyeloplasty in patients with UPJO. Published material was identified utilizing the PubMed® (National Center for Biotechnology Information, United States National Library of Medicine, National Institutes of Health) database using multiple combinations of the keywords: ureteropelvic junction obstruction in children, pediatric robot-assisted laparoscopic pyeloplasty, pediatric robotic pyeloplasty, pediatric robot-assisted dismembered pyeloplasty, robotic surgery cost in children, cost analysis, learning curve of robotic surgery in children, robotic simulation, weight and age in pediatric robotic pyeloplasty, future of robotics in children. The articles were then read for relevancy and the bibliographies reviewed for additional citations. Article inclusion within this review was determined by the authors after their evaluation, analysis, and interpretation.
Discussion
Ureteropelvic junction obstruction (UPJO)
Development of prenatal screening ultrasonography (US) and postnatal imaging have increased the diagnosis of hydronephrosis and, consequently, decreased the age of pyeloplasty. Prenatal hydronephrosis has been found in up to 4.5% screening ultrasounds, while UPJO occurs in 1 per 1000–2000 newborns [9]. UPJO has a male predominance and affects the left kidney in up to 67% of cases, while up to 10% of cases are bilateral [9]. An ultrasound (US) of the urinary tract allows to grade the severity of hydronephrosis and to determine pelvocalyceal dilation and/or renal cortical thinning at birth. In 1993, the Society for Fetal Urology (SFU) suggested a standard US grading system for the evaluation of hydronephrosis, according to urinary tract dilatation and parenchymal thickness [9]. In 2014, the urinary tract dilation (UTD) classification system was introduced, and six parameters started to be evaluated: anterior–posterior renal pelvic diameter, urinary tract dilatation, parenchymal thickness, parenchymal appearance, ureteral status, and bladder status [10]. It should be emphasized that the first postnatal US should be done more than 48 h after birth to ensure it does not underestimate dilation [1]. In addition to US, 99mTc-MAG3 is recommended for neonatal renography and for visualization of kidneys in patients with compromised renal function [10]. However, being functionally immature, neonatal kidneys may show increased residual cortical activity with the simultaneous injection of the radiopharmaceutical and furosemide (F0 study), retaining up to 50% or more of the peak. Such a phenomenon disappears after the age of 3 months.
Indications
The indications of RALP do not differ from those of open or laparoscopic surgery and include symptomatic obstruction, urinary tract infections, presence of an obstructive pattern on functional renal scan, and/or worsening differential renal function (DRF) [10]. To date, de Waard et al. in 2018 speculated that hypertension should be considered an indication for surgery as the relief of the obstruction cures hypertension in most children with UPJO [11]. A recent study showed that preoperative characteristics, including sex distribution, laterality, preoperative renal pelvis antero-posterior (AP) diameter, and the split functions of the affected kidneys are comparable between LP and RALP [10].
Real concerns, however, have been raised on performing the procedure in very small spaces [12, 13] and its relative value in small children, as well as the influence of body weight on the outcome in children treated with robotic pyeloplasty, since at present, no specific robotic devices have been developed exclusively for pediatric surgery. Although the consensus goes toward a weight cutoff at 10–15 kg, some authors have reported the successful use of robotic technology in patients under 1 year of age and in infants [13,14,15]. Another topic supporting the limitation of RALP in small children consists of trocar placement due to the restricted space that often causes conflicts between arms. A recent paper has proved the efficacy of 5 mm rather than 8 mm instruments to optimize the available working space [16,17,18]. However, since monopolar curved scissors are not available for use with 5 mm ports, monopolar hook diathermy is the alternative. Notwithstanding the weight, correct bed positioning, a reproducible trocar placement method (‘kite-like’ configuration), and the use of a ‘tent effect’ seem sufficient to achieve an adequate working space and avoid prolonged operative setup and operative times [13]. Data on younger and lighter infants are encouraging despite all the studies being mostly retrospective.
Surgical technique and operative time
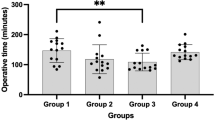
The preferred approach to UPJO is the dismembered pyeloplasty described by Anderson–Hynes. While the most used approach is the transperitoneal [19,20,21,22], some authors advocate the use of a retroperitoneal approach [23, 24]. A recent multicenter prospective study [25] comparing transperitoneal versus retroperitoneal RALP showed that both approaches are safe and effective, with a shorter hospital stay, but with longer operative time in case of crossing vessels for retroperitoneal RALP. However, a univocal consensus on the approach is yet to be defined and remains a matter of personal preference. While a 3–5 cm distance between ports is ideal, in infants this distance is not always possible, so the trocars are placed as far apart as possible. In transabdominal RALP, ports are positioned as follows: one infraumbilical robotic camera port; two 8 mm working ports along the midclavicular line, one in the epigastric region, 2 cm under the subcostal arch, the other in the right/left iliac fossa, above the inguinal ligament; one 5 mm assistant port between the optic port and the lower working port (left pyeloplasties) or between the optics and the upper working port to lift the liver (right pyeloplasties) [25, 26]. For retroperitoneal RALP, ports positioning has been standardized as well [23, 24]. Recently, robot-assisted laparoscopic single-port pyeloplasty has shown to be feasible in noninfant pediatric patients [27, 28]. Of note, new hidden incision endoscopic surgery (HIdES) technique aims to eliminate visible scarring, placing the robotic working port and camera port below the line of a Pfannenstiel incision, while a second working port is placed infraumbilically [29]. A recent comparative cross-sectional study [30] using da Vinci ** Surgical System® compared the efficacy, safety, and cosmetic outcomes of three-port RALP with the conventional four-port RALP method, showing that the first can be applied with similar success and safety to the latter in all patients, including infants. Three-port RALP involves the use of a percutaneous hitch stitch to hold the renal pelvis and a 14-G angiocatheter via the percutaneous route to place the double-J stent. Concerning the operative time (OT), the experience acquired through the years has shortened the overall OT to the extent that, from 2019, most studies have always reported an overall OT of less than 120 min [7, 12, 13, 31, 32]. Currently under debate is the necessity for urinary diversion with trans-anastomotic ureteral stenting during pyeloplasty. Stenting is mostly performed through double-J stent placed in an antegrade fashion, or cutaneous pyeloureteral (CPU) stents designed to provide effective urinary drainage, prevent secondary anesthesia, prevent bleeding risks, and be cost-effective [33]. However, since RALP has been extended to smaller children and infants, which have shown a higher risk of stent-related complications (stent migration and fragmentation, infection, fever, pain) and discomfort, surgeons have started to perform robotic stentless pyeloplasty [34,35,36], showing excellent success rates and minimal complications compared to conventional methods, but studies on larger cohorts and with longer follow-up are needed.
Outcomes
Pediatric RALP success rate ranges from 90 to 100% [20, 22, 24, 32, 36,37,38,39,40,41,42,43,44,45,46,47]. In comparison to open or laparoscopic pyeloplasty, RALP presents a shorter hospital stay and less use of medication for pain management following the procedure. What was a negative variable before, namely the longer operative times compared to other modalities, is constantly improving above all in centers with high volume and surgeon experience. Early postoperative complications (< 30 days) range from fever, pain, and hematuria (Clavien I) to urinary tract infection, mild or major urinary leaks (Clavien ≥ 2), to stent migration, omental herniation, and ileocecal volvulus (Clavien IIIb) [19, 39, 41, 48]. Late (≥ 30 days) complications were reported in few cases through the literature and were mostly Clavien IIIb (ipsilateral ureteral stenosis, surgical interventions for umbilical hernia, J–J migration, urinary leakage, and stone formation) [48,49,50].
Redo RALP
The incidence of persistent or recurrent UPJO after initial pyeloplasty can range from 3 to 11% [51] and there is no consensus regarding the gold standard approach for failed pyeloplasty. Interestingly, literature has shown some qualitative differences between indications for secondary procedures between laparoscopic and robotic pyeloplasty: the first causes more frequently urine leaks, while RALP has complications related to retrograde pyelogram (RPG) and stent placement, possibly because of its technical ease of intracorporeal suturing [52]. In general, the reason for redo pyeloplasty is obstruction due to crossing vessel, intrinsic narrowing, or high insertion and patients often undergo various endoscopic interventions (placement of stents or percutaneous nephrostomy tube (PCN), endopyelotomy, balloon dilation) without resolution [53]. Of note, RALP simplifies the visualization of crossing vessels and their concomitant surgical management. Despite that the management of UJPO recurrence is more challenging and reserved to the most experienced surgeons due to scar tissue formation, fibrosis, and decreased vascularization of the ureter tract, redo RALP is considered an efficient and safe approach with shorter hospital stay and lower complication rate compared to redo open pyeloplasty and a high success rate similar to the outcomes of primary RALP [53,54,55,56,57,58,59,60].
Challenging cases
Some cases are characterized by complex anatomy and require experienced hands for the dissection of the UPJ without causing vascular lesions. They include complete intrarenal pelvis, high ureteral insertion, or long ureteral stricture, as well as anatomic variations of morphology and position of the kidney as horseshoe kidney (HSK), renal malrotation, ectopic kidney, or duplex kidney with lower moiety UPJO. Esposito et al. [61] gathered the widest series on complex cases in a multicentric study recommending the use of Anderson–Hynes technique with da Vinci **, as it can easily adapt to: vascular anatomy of UPJ in patients with HSK and ectopic kidney, presence of crossing vessels, cases with lower pole UPJO in which the vascularity of the upper moiety ureter should be carefully preserved to avoid stenosis, as well scar fibrotic tissues for recurrent UPJO after failed open pyeloplasty. With a special eye on HSK, which has an increased incidence of UPJO for high insertion of the ureter into the renal pelvis, anatomic relation of the ureter with HSK isthmus, multiple aberrant crossing vessels, urolithiasis, and an increased risk of trauma, a recent multicentric study by the same group [62] showed RALP safety, feasibility and good medium-term outcomes, with an average operative time including docking of 143.5 min (range 100–205), no conversions to laparoscopy or open surgery, no intraoperative complications and overall success rate of 92.8%.
In the adult population, robot-assisted laparoscopic surgery (RALS) has been successfully employed for the treatment of renal stones during concomitant treatment of UPJO and for the primary treatment of staghorn stones [63]. Reports on children with concomitant UPJO and large renal stones [64, 65] demonstrate that simultaneous pyelolithotomy and pyeloplasty in a single surgical session is safe and feasible, despite EAU guidelines recommendation that RALP and surgical treatment for stones should be performed in separate procedures [66].
Cost considerations
RALP is more expensive than an open procedure, although this cost differential decreased with time and institutional experience. In 2016, Bennett et al. [67] found that charges for operative room time and supplies together with anesthesia time dominate the cost difference between RALP and open pyeloplasty, and that efforts to reduce these specific costs should be the focus of future cost-containment measures. A recent work published in 2021 [68] confirmed a similar cost burden of operating theater, instruments, material, and ward convalescence between open and RALP and justified the use of RALP in a low-volume center. The procedure required for double-J stent removal represented an additional cost. However, in this series, the use of magnetic stents, which avoided the need for further anesthetic procedures, limited costs. Basic cost analysis has shown similar cost between robotic and traditional laparoscopic pyeloplasty [69,70,71,72]. However, the high cost of training, maintenance, and materials point to a greater cost for RALP as compared to other modalities [73]. Strategies to lower cost and raise the value of care have been proposed and include: increasing robot utilization, lowering OR turnover time, and optimizing preoperative holding time. All in all, direct costs of RALP, excluding amortization, robotic cost, maintenance, and depreciation, should not be considered as an excessive burden compared to other surgical modalities.
Training and learning curve
In a meta-analysis by Steinberg et al., the authors found that the learning curve for robotic prostatectomy in adults ranged from 13 to 200 cases, with 77 being the average [74]. Also in pediatric robotics, unfortunately, a standardized robotic curriculum or training protocol is still not available. An open surgeon can quickly attain expertise in RALP by working with a proctor and experienced surgical team [75], but the duration of proctoring needed will vary by individual surgeons and therefore critical self-assessment is essential. Skill acquisition in laparoscopy and robotic pyeloplasty is different in the rate of progression toward proficiency: one study demonstrated that at least 18 cases were required to achieve proficiency in LP, while 13 cases were needed for RALP, with further improvement after 37 cases [45, 46]. Sorensen et al. observed that operative times for RALP were initially longer than open, but became equivalent after 15–20 cases, suggesting that this is the approximate length of the initial learning curve for RALP [76]. Recently, Esposito et al. [7] experienced a learning curve plateau after the first 23 consecutive cases. Moreover, it has been shown how junior surgeons can readily achieve comparable levels of expertise compared with senior practitioners for RALP, assuming proper exposure to robotics and an adequate case volume [77]. A key role should be played by robotic simulation, allowing trainees to learn the basic controls of the instrument and practice surgical skills, as it happens in laparoscopy [77].
Future considerations
The use of robotic technology has grown in pediatric urology and likely will continue to do so in the future to potentially become the gold standard for certain reconstructive cases, especially RALP. To date, efforts have been made to produce 5 mm instruments, which, however, still have a limited selection, require more working space due to typical joint kinematics, and imply the use of a 5 mm lens that removes the advantageous 3-D image and the 5 mm instruments. For this reason, some authors advocate efforts to improve the quality of 5 mm instruments or to develop 3 mm instruments similar to those used in conventional laparoscopy. Moreover, future trend appears to be moving toward less incisions down to a single-port platform and possibly even no incision in the future [78]. Considering innovation and technology development, augmented reality (AR) and augmented intelligence might represent the next steps in robotic surgery [79, 80].
Conclusions
RALP demonstrated to be safe and effective, also in children with recurrent UPJO, where it allows an easy identification and consequent dissection of the causes of the initial failed reconstruction, including missed crossing vessels, periureteral fibrosis, and ureteral stricture, as well as in complex anatomical cases [60, 61, 64]. Moreover, RALP shortens the learning curve for junior surgeons, who can readily achieve levels of expertise comparable to senior practitioners [76]. The high cost remains a limitation of robotic surgery, which is the reason why robotic instrumentation is shared between various sub-specialties. However, if from one side this contributes to cost limitation, from the other it reduces the learning curve process. In fact, simulation alone is not sufficient to reach proficiency and a comprehensive training program should consist of simulation and mentorship. The main disadvantage of RALP is the size of robotic instruments, which cause difficulties in the treatment of small children and infants. Despite that some surgeons can overcome this drawback by using 5 mm instruments and others by adjusting port positioning, the final choice should be tailored on surgeon’s preference and customized according to the specific patient. Further high-quality prospective observational studies and clinical trials on RALP in small children, the creation of a pediatric robotic curriculum for learning curve, as well as new technologies and instruments specific for the pediatric population are advisable for RALP to reach the level of gold standard.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Li B, McGrath M, Farrokhyar F et al (2020) Ultrasound-based scoring system for indication of pyeloplasty in patients with UPJO-like hydronephrosis. Front Pediatr 8:353. https://doi.org/10.3389/fped.2020.00353
Salö M, Sjöberg Altemani T, Anderberg M (2016) Pyeloplasty in children: perioperative results and long-term outcomes of robotic-assisted laparoscopic surgery compared to open surgery. Pediatr Surg Int 32(6):599–607. https://doi.org/10.1007/s00383-016-3869-2
Chan YY, Durbin-Johnson B, Sturm RM, et al (2017) Outcomes after pediatric open, laparoscopic, and robotic pyeloplasty at Academic Institutions. J Pediatr Urol 13(1):49.e1–49.e6 https://doi.org/10.1016/j.jpurol.2016.08.029
Tomaszewski JJ, Casella DP, Turner RM et al (2012) Pediatric laparoscopic and robot-assisted laparoscopic surgery: technical considerations. J Endourol 26(6):602–613. https://doi.org/10.1089/end.2011.0252
Lee RS, Retik AB, Borer JG, et al (2006) Pediatric robot assisted laparoscopic dismembered pyeloplasty: comparison with a cohort of open surgery. J Urol 175(2):683–7; discussion 687 https://doi.org/10.1016/S0022-5347(05)00183-7
Peters CA (2004) Robotically assisted surgery in pediatric urology. Urol Clin North Am 31(4):743–752. https://doi.org/10.1016/j.ucl.2004.06.007
Esposito C, Masieri L, Castagnetti M, et al (2019) Robot-assisted vs laparoscopic pyeloplasty in children with uretero-pelvic junction obstruction (UPJO): technical considerations and results. J Pediatr Urol 15(6):667.e1–667.e8 https://doi.org/10.1016/j.jpurol.2019.09.018
Muneer A, Arya M, Shergill IS et al (2008) Current status of robotic surgery in pediatric urology. Pediatr Surg Int 24(9):973–977. https://doi.org/10.1007/s00383-008-2208-7
Vemulakonda VM, Wilcox DT, Crombleholme TM et al (2015) Factors associated with age at pyeloplasty in children with ureteropelvic junction obstruction. Pediatr Surg Int 31(9):871–877. https://doi.org/10.1007/s00383-015-3748-2
Silay MS, Spinoit AF, Undre S, et al (2016) Global minimally invasive pyeloplasty study in children: results from the pediatric urology expert group of the european association of urology young academic urologists working party. J Pediatr Urol 12(4):229.e1–7 https://doi.org/10.1016/j.jpurol.2016.04.007
Thakre AA, Bailly Y, Sun LW, et al (2008) Is smaller workspace a limitation for robot performance in laparoscopy? J Urol 179(3):1138–42; discussion 1142–3 https://doi.org/10.1016/j.juro.2007.10.091
Masieri L, Sforza S, Grosso AA, et al (2020) Does the body weight influence the outcome in children treated with robotic pyeloplasty? J Pediatr Urol 16(1):109.e1–109.e6 https://doi.org/10.1016/j.jpurol.2019.10.023
Kafka IZ, Kocherov S, Jaber J et al (2019) Pediatric Robotic-Assisted Laparoscopic Pyeloplasty (RALP): does weight matter? Pediatr Surg Int 35(3):391–396. https://doi.org/10.1007/s00383-019-04435-y
Kawal T, Srinivasan AK, Shrivastava D, et al (2018) Pediatric robotic-assisted laparoscopic pyeloplasty: does age matter? J Pediatr Urol 14(6):540.e1–540.e6 https://doi.org/10.1016/j.jpurol.2018.04.023
Bansal D, Cost NG, DeFoor WR Jr et al (2014) Infant robotic pyeloplasty: comparison with an open cohort. J Pediatr Urol 10(2):380–385. https://doi.org/10.1016/j.jpurol.2013.10.016
Ganpule A, Jairath A, Singh A et al (2015) Robotic versus conventional laparoscopic pyeloplasty in children less than 20 Kg by weight: single-center experience. World J Urol 33(11):1867–1873. https://doi.org/10.1007/s00345-015-1694-1
Kawal T, Sahadev R, Srinivasan A et al (2020) Robotic surgery in infants and children: an argument for smaller and fewer incisions. World J Urol 38(8):1835–1840. https://doi.org/10.1007/s00345-019-02765-z
Ballouhey Q, Villemagne T, Cros J et al (2015) A comparison of robotic surgery in children weighing above and below 15.0 Kg: size does not affect surgery success. Surg Endosc 29(9):2643–2650. https://doi.org/10.1007/s00464-014-3982-z
Neheman A, Kord E, Zisman A et al (2018) Comparison of robotic pyeloplasty and standard laparoscopic pyeloplasty in infants: a bi-institutional study. J Laparoendosc Adv Surg Tech A 28(4):467–470. https://doi.org/10.1089/lap.2017.0262
Atug F, Woods M, Burgess SV, et al (2005) Robotic assisted laparoscopic pyeloplasty in children. J Urol;174(4 Part 1):1440–1442 https://doi.org/10.1097/01.ju.0000173131.64558.c9
Kutikov A, Nguyen M, Canter T (2006) Robot assisted pyeloplasty in the infant-lessons learned. J Urol 176:2239–2240
Yee DS, Shanberg AM, Duel BP et al (2006) Initial comparison of robotic-assisted laparoscopic versus open pyeloplasty in children. Urology 67(3):599–602. https://doi.org/10.1016/j.urology.2005.09.021
Blanc T, Kohaut J, Elie C et al (2019) Retroperitoneal approach for ureteropelvic junction obstruction: encouraging preliminary results with robot-assisted laparoscopic repair. Front Pediatr 7:209. https://doi.org/10.3389/fped.2019.00209
Olsen LH, Rawashdeh YF, Jorgensen TM (2007) Pediatric robot assisted retroperitoneoscopic pyeloplasty: a 5-year experience. J Urol 178:2137
Blanc T, Abbo O, Vatta F et al (2022) Transperitoneal versus retroperitoneal robotic-assisted laparoscopic pyeloplasty for ureteropelvic junction obstruction in children. A multicentre, prospective study. Eur Urol Open Sci 41:134–140. https://doi.org/10.1016/j.euros.2022.05.009
Kim SJ, Barlog JS, Akhavan A (2018) Robotic-assisted urologic surgery in infants: positioning, trocar placement, and physiological considerations. Front Pediatr 6:411. https://doi.org/10.3389/fped.2018.00411
Kang SK, Jang WS, Kim SH et al (2021) Comparison of intraoperative and short-term postoperative outcomes between robot-assisted laparoscopic multi-port pyeloplasty using the Da Vinci si system and single-port pyeloplasty Using the Da Vinci SP system in children. Investig Clin Urol 62(5):592–599. https://doi.org/10.4111/icu.20200569
Chandrasoma S, Kokorowski P, Peters CA et al (2010) Straight-arm positioning and port placement for pediatric robotic-assisted laparoscopic renal surgery. J Robot Surg 4(1):29–32. https://doi.org/10.1007/s11701-010-0184-0
Hong YH, DeFoor WR Jr, Reddy PP et al (2018) Hidden Incision Endoscopic Surgery (HIdES) trocar placement for pediatric robotic pyeloplasty: comparison to traditional port placement. J Robot Surg 12(1):43–47. https://doi.org/10.1007/s11701-017-0684-2
Danacioglu YO, Keser F, Polat S et al (2022) Assistant port is unnecessary for robotic-assisted laparoscopic pyeloplasty in children: a comparative cohort study. Pediatr Surg Int. https://doi.org/10.1007/s00383-022-05158-3
Sforza S, Di Maida F, Mari A et al (2019) Is a drainage placement still necessary after robotic reconstruction of the upper urinary tract in children? experience from a tertiary referral center. J Laparoendosc Adv Surg Tech A 29(9):1180–1184. https://doi.org/10.1089/lap.2019.0302
Silay MS, Danacioglu O, Ozel K et al (2020) Laparoscopy versus robotic-assisted pyeloplasty in children: preliminary results of a pilot prospective randomized controlled trial. World J Urol 38(8):1841–1848. https://doi.org/10.1007/s00345-019-02910-8
Dangle PP, Shah AB, Gundeti MS (2014) Cutaneous pyeloureteral stent for laparoscopic (Robot)-assisted pyeloplasty. J Endourol 28(10):1168–1171. https://doi.org/10.1089/end.2013.0499
Rodriguez AR, Rich MA, Swana HS (2012) Stentless pediatric robotic pyeloplasty. Ther Adv Urol 4(2):57–60. https://doi.org/10.1177/1756287211434927
Casale P, Lambert S (2010) Prospective analysis of completely stentless robot-assisted pyeloplasty in children. J Robot Surg 3(4):215–217. https://doi.org/10.1007/s11701-009-0164-4
Silva MV, Levy AC, Finkelstein JB, et al (2015) Is peri-operative urethral catheter drainage enough? The case for stentless pediatric robotic pyeloplasty. J Pediatr Urol 11(4):175.e1–e5 https://doi.org/10.1016/j.jpurol.2015.06.003.
Freilich DA, Nguyen HT, Borer J et al (2008) Concurrent management of bilateral ureteropelvic junc- tion obstruction in children using robotic-assisted laparo- scopic surgery. Int Braz J Urol 34:204–205
Minnillo BJ, Cruz JAS, Sayao RH et al (2011) Long-term experience and outcomes of robotic assisted laparoscopic pyeloplasty in children and young adults. J Urol 185(4):1455–1460. https://doi.org/10.1016/j.juro.2010.11.056
Ng D, Reddy PP, Noh PH (2013) Pediatric standard and robot-assisted laparoscopic pyeloplasty: a comparative single institution study. J Urol 189:283–287
Dangle PP, Kearns J, Anderson B et al (2013) Out- comes of infants undergoing robot-assisted laparoscopic pyeloplasty compared to open repair. J Urol 190:2221–2226
Bansal D, Cost NG, Defoor WR, Vanderbrink BA et al (2014) Infant robotic pyeloplasty: comparison with an open cohort. J Pediatr Urol 10:380–385
Radford A, Turner A, Ashraf J et al (2018) Robotic pyeloplasty in children: a “Barbed” shortcut. J Laparoendosc Adv Surg Tech A 28(4):486–489. https://doi.org/10.1089/lap.2017.0494
Junejo NN, Alotaibi A, Alshahrani SM et al (2020) The learning curve for robotic-assisted pyeloplasty in children: our initial experience from a single center. Urol Ann 12(1):19–24. https://doi.org/10.4103/UA.UA_113_19
Li P, Zhou H, Cao H et al (2021) Early robotic-assisted laparoscopic pyeloplasty for infants under 3 months with severe ureteropelvic junction obstruction. Front Pediatr 9:590865. https://doi.org/10.3389/fped.2021.590865
Liu Y, Wu M, Wang W et al (2021) Retrospective analysis of the efficacy of Da Vinci robot-assisted pyeloplasty in the treatment of ureteropelvic junction obstruction in children. J Healthc Eng. https://doi.org/10.1155/2021/5398858
Andolfi C, Lombardo AM, Aizen J et al (2022) Laparoscopic and robotic pyeloplasty as minimally invasive alternatives to the open approach for the treatment of uretero-pelvic junction obstruction in infants: a multi-institutional comparison of outcomes and learning curves. World J Urol 40(4):1049–1056. https://doi.org/10.1007/s00345-022-03929-0
Salö M, Bonnor L, Graneli C et al (2022) Ten years of paediatric robotic surgery: lessons learned. Int J Med Robot 18(4):e2386. https://doi.org/10.1002/rcs.2386
Avery DI, Herbst KW, Lendvay TS, et al (2015) Robot-assisted laparoscopic pyeloplasty: multi-institutional experience in infants. J Pediatr Urol 11(3):139.e1–e5 https://doi.org/10.1016/j.jpurol.2014.11.025.
Murthy P, Cohn JA, Gundeti MS (2015) Evaluation of robotic- assisted laparoscopic and open pyeloplasty in children: single- surgeon experience. Ann R Coll Surg Engl 97:109–114
Subotic U, Rohard I, Weber DM et al (2012) A minimal invasive surgical approach for children of all ages with ureteropelvic junction obstruction. J Pediatr Urol 8(4):354–358. https://doi.org/10.1016/j.jpurol.2011.07.004
Dy GW, Hsi RS, Holt SK et al (2016) National trends in secondary procedures following pediatric pyeloplasty. J Urol 195(4 Pt 2):1209–1214. https://doi.org/10.1016/j.juro.2015.11.010
Ebert KM, Nicassio L, Alpert SA, et al (2020) Surgical outcomes are equivalent after pure laparoscopic and robotic-assisted pyeloplasty for ureteropelvic junction obstruction. J Pediatr Urol 16(6):845.e1–845.e6 https://doi.org/10.1016/j.jpurol.2020.09.018
Mittal S, Aghababian A, Eftekharzadeh S, et al (2021) Primary vs redo robotic pyeloplasty: a comparison of outcomes. J Pediatr Urol 17(4):528.e1–528.e7 https://doi.org/10.1016/j.jpurol.2021.02.016.
Zhang Y, Ouyang W, Xu H et al (2019) Secondary management for recurrent ureteropelvic junction obstruction after pyeloplasty: a comparison of Re-Do robot-assisted laparoscopic pyeloplasty and conventional laparoscopic pyeloplasty. Urol Int 103(4):466–472. https://doi.org/10.1159/000503156
Jacobson DL, Shannon R, Johnson EK et al (2019) Robot-assisted laparoscopic reoperative repair for failed pyeloplasty in children: an updated series. J Urol 201(5):1005–1011. https://doi.org/10.1016/j.juro.2018.10.021
Baek M, Silay MS, Au JK et al (2018) Quantifying the additional difficulty of pediatric robot-assisted laparoscopic re-do pyeloplasty: a comparison of primary and re-do procedures. J Laparoendosc Adv Surg Tech A 28(5):610–616. https://doi.org/10.1089/lap.2016.0691
Davis TD, Burns AS, Corbett ST, et al (2016) Reoperative Robotic pyeloplasty in children. J Pediatr Urol 12(6):394.e1–394.e7 https://doi.org/10.1016/j.jpurol.2016.04.045
Asensio M, Gander R, Royo GF, et al (2015) Failed pyeloplasty in children: is robot-assisted laparoscopic reoperative repair feasible? J Pediatr Urol 11(2):69.e1–6 https://doi.org/10.1016/j.jpurol.2014.10.009.
Lindgren BW, Hagerty J, Meyer T et al (2012) Robot-assisted laparoscopic reoperative repair for failed pyeloplasty in children: a safe and highly effective treatment option. J Urol 188(3):932–937. https://doi.org/10.1016/j.juro.2012.04.118
Hemal AK, Mishra S, Mukharjee S et al (2008) Robot assisted laparoscopic pyeloplasty in patients of ureteropelvic junction obstruction with previously failed open surgical repair: robotic pyeloplasty in failed UPJ repair. Int J Urol 15(8):744–746. https://doi.org/10.1111/j.1442-2042.2008.02091.x
Esposito C, Masieri L, Blanc T et al (2021) Robot-Assisted Laparoscopic Pyeloplasty (RALP) in children with complex Pelvi-Ureteric Junction Obstruction (PUJO): results of a multicenter European report. World J Urol 39(5):1641–1647. https://doi.org/10.1007/s00345-020-03331-8
Esposito C, Masieri L, Blanc T et al (2019) Robot-Assisted Laparoscopic Pyeloplasty (RALP) in Children with Horseshoe Kidneys: Results of a Multicentric Study. World J Urol. https://doi.org/10.1007/s00345-019-02632-x
Jensen PH, Berg KD, Azawi NH (2017) Robot-assisted pyeloplasty and pyelolithotomy in patients with ureteropelvic junction stenosis. Scand J Urol 51(4):323–328 https://doi.org/10.1080/21681805.2017.1300188.
Passerotti Cc Cendron M, Borer JG, Peters CA (2007) Early results of robot assisted laparoscopic li- thotomy in adolescents. J Urol 177:2309–2310
Esposito C, Masieri L, Blanc T et al (2021) Robot-assisted laparoscopic surgery for treatment of urinary tract stones in children: report of a multicenter international experience. Urolithiasis 49(6):575–583. https://doi.org/10.1007/s00240-021-01271-5
Ballesteros N, Snow ZA, Moscardi PRM et al (2019) Robotic management of urolithiasis in the pediatric population. Front Pediatr 7:351. https://doi.org/10.3389/fped.2019.00351
Bennett WE, Whittam BM, Szymanski KM et al (2017) Validated cost comparison of open vs. robotic pyeloplasty in American Children’s Hospitals. J Robot Surg 11(2):201–206. https://doi.org/10.1007/s11701-016-0645-1
Pakkasjärvi N, Taskinen S (2021) Introduction of pediatric robot-assisted pyeloplasty in a low-volume centre. Clin Pract 11(1):143–150. https://doi.org/10.3390/clinpract11010020
Casella DP, Fox JA, Schneck FX et al (2013) Cost analysis of pediatric robot-assisted and laparoscopic pyeloplasty. J Urol 189(3):1083–1086. https://doi.org/10.1016/j.juro.2012.08.259
Cundy TP, Harling L, Hughes-Hallett A et al (2014) Meta-analysis of robot-assisted vs conventional laparoscopic and open pyeloplasty in children: robot-assisted vs laparoscopic and open pyeloplasty in children. BJU Int 114(4):582–594. https://doi.org/10.1111/bju.12683
Varda BK, Johnson EK, Clark C et al (2014) National trends of perioperative outcomes and costs for open, laparoscopic and robotic pediatric pyeloplasty. J Urol 191(4):1090–1095. https://doi.org/10.1016/j.juro.2013.10.077
Varda BK, Wang Y, Chung BI, et al (2018) Has the robot caught up? National trends in utilization, perioperative outcomes, and cost for open, laparoscopic, and robotic pediatric pyeloplasty in the United States from 2003 to 2015. J Pediatr Urol 14(4):336.e1–336.e8 https://doi.org/10.1016/j.jpurol.2017.12.010
Bodar YJL, Srinivasan AK, Shah AS, et al (2020) Time-driven activity-based costing identifies opportunities for process efficiency and cost optimization for robot-assisted laparoscopic pyeloplasty. J Pediatr Urol 16(4):460.e1–460.e10 https://doi.org/10.1016/j.jpurol.2020.05.146.
Steinberg PL, Merguerian PA, Bihrle W et al (2008) The cost of learning robotic-assisted prostatectomy. Urology 72(5):1068–1072. https://doi.org/10.1016/j.urology.2007.11.118
Bowen DK, Lindgren BW, Cheng EY et al (2017) Can proctoring affect the learning curve of robotic-assisted laparoscopic pyeloplasty? Experience at a high-volume pediatric robotic surgery center. J Robot Surg 11(1):63–67. https://doi.org/10.1007/s11701-016-0613-9
Sorensen MD, Delostrinos C, Johnson MH et al (2011) Comparison of the learning curve and outcomes of robotic assisted pediatric pyeloplasty. J Urol 185(6 Suppl):2517–2522. https://doi.org/10.1016/j.juro.2011.01.021
Spampinato G, Binet A, Fourcade L et al (2021) Comparison of the learning curve for robot-assisted laparoscopic pyeloplasty between senior and junior surgeons. J Laparoendosc Adv Surg Tech A 31(4):478–483. https://doi.org/10.1089/lap.2020.0822
Lendvay TS, Hannaford B, Satava RM (2013) Future of robotic surgery. Cancer J 19(2):109–119. https://doi.org/10.1097/PPO.0b013e31828bf822
Sheth KR, Koh CJ (2019) The future of robotic surgery in pediatric urology: upcoming technology and evolution within the field. Front Pediatr 7:259. https://doi.org/10.3389/fped.2019.00259
Meinzer A, Alkatout I, Krebs TF et al (2020) Advances and trends in pediatric minimally invasive surgery. J Clin Med 9(12):3999. https://doi.org/10.3390/jcm9123999
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
C.E. and M.C. designed the study, coordinated the team and reviewed the manuscript; M.E., R.C. and B. L. drafted the manuscript; V. C., F. D.C., G.E. and D.DA performed data analysis. All authors had access to the study data, reviewed and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esposito, C., Cerulo, M., Lepore, B. et al. Robotic-assisted pyeloplasty in children: a systematic review of the literature. J Robotic Surg 17, 1239–1246 (2023). https://doi.org/10.1007/s11701-023-01559-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-023-01559-1