Abstract
The pathophysiology of migraine as a headache disorder is still undetermined. Diffusion tensor imaging (DTI) has significantly improved our knowledge about brain microstructure in this disease. Here, we aimed to systematically review DTI studies in migraine and survey the sources of heterogeneity by investigating diffusion parameter changes associated with clinical characteristics and migraine subtypes. Microstructural changes, as revealed by widespread alteration of diffusion metrics in white matter (WM) tracts, subcortical and cortical regions, were reported by several migraine DTI studies. Specifically, we reported changes in the corpus callosum, thalamic radiations, corona radiata, and brain stem. These alterations showed high variability across migraine cycle phases. Additionally, migraine associated with depressive/anxiety symptoms revealed significant changes in the corpus callosum, internal capsule, and superior longitudinal fasciculus. No significant WM microstructural differences were observed between migraine patients with and without aura. Overall, differences between chronic and episodic migraine showed inconsistency across studies. Migraine is associated with microstructural changes in widespread regions including thalamic radiations, corpus callosum, and brain stem. These alterations can highlight neuronal damage and neuronal plasticity mechanisms either following pain stimulations occurring in migraine cycle or as a compensatory response to pain in chronic migraine. Longitudinal studies applying advanced modalities may shed new light on the underlying microstructural changes in migraine subtypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a major neurological disorder characterized by moderate or severe headaches with a unilateral, pulsatile quality accompanied by a myriad of symptoms, such as nausea, photophobia, and phonophobia. There are several categorizations for migraine, such as chronic (CM) and episodic migraine (EM), migraine with aura (MWA), and migraine without aura (MWoA), i.e., the presence/absence of sensory disturbances, including flashes of light, blind spots, and hand/face tingling, respectively ("Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition," 2018). According to the global burden of disease study in 2017, headache disorders were the second most prevalent disease and the second-highest contributor to age-standardized global years lost due to disability (YLD). Among headache disorders, migraine was in the first rank based on YLD and in the second rank based on prevalence (Sevenich, 2018). Despite the high prevalence and the well-known clinical features of this disease, the cascade of events triggering initiation and disease progression is far from being understood.
Recently, it has been suggested that alterations in mechanisms involved in cortical excitatory-inhibitory balance may promote hyper-reactivity to pain in individuals genetically susceptible to migraine (Gasparini et al., 2013; Mainero et al., 2011). Because of this increased neuronal excitation, a wave of cortical spreading depression (CSD) turns up and spreads across the cerebral cortex (Charles & Baca, 2013; Granziera et al., 2006). Animal studies showed that neuronal excitation and the subsequent CSD might lead to several alterations, such as activation of trigeminal afferent neurons (Karatas et al., 2013; Moskowitz et al., 1993), increasing brain vascular permeability, and promoting neuroinflammation (Cutrer et al., 2012; Gursoy-Ozdemir et al., 2004). The latter can trigger neuronal firing in the spinal and trigeminal nucleus and in the meningeal nociceptors (Kincses et al., 2019). Thus, the recurrent sensitization of the trigeminovascular system linked with the increased reactivity to stimuli is considered the main trigger of the cascade of events associated with migraine attacks (DaSilva et al., 2007; Welch, 2005).
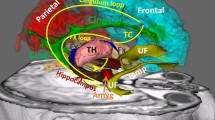
The trigeminal complex projects to the brainstem, hypothalamus, basal ganglia, thalamic, and cortical regions involved in sensory and cognitive pain input and aura (Dodick, 2018; Noseda et al., 2011). Projection neurons can modulate central signals from the periaqueductal gray matter (PAG), dorsolateral pons, medullary raphe, spinal trigeminal nucleus (SpV), as well as the descending cortical inhibitory complex (Marciszewski et al., 2018). These areas mediate the intensity of sensory stimuli, cerebral blood flow, and nociception of cortical and subcortical neurons (Dodick, 2018; Maniyar et al., 2014) exhibiting different levels of activity in the migraine stages (Dodick, 2018; Moulton et al., 2008). Similarly, the thalamus, a bilateral brain structure projecting out to the cerebral cortex through several tracts, such as the fornix, cingulum, anterior thalamic radiations (ATR) and posterior thalamic radiations (PTR) (Jones, 2002; Zhang et al., 2010), is involved in the pathophysiology of migraine (Li et al., 2011; Yuan et al., 2012).
Despite these significant advancements in the comprehension of the potential neural mechanisms linked with migraine, we still lack effective imaging biomarkers aimed at predicting the disease course or treatment response (Dodick, 2018). In this regard, the analysis of the human connectome, i.e., the human brain organization into highly interconnected regions (Sporns et al., 2005), could help identify new non-invasive cost-effective biomarkers for diagnosis, progression, and as surrogate outcomes for clinical trials (Dodick, 2018; Katsarava et al., 2012). In the last years, diffusion-weighted imaging (DWI) sequences, based on the difference in magnitude of water diffusion, have improved our knowledge of neurological disorders. Diffusion tensor imaging (DTI) comprises a group of techniques computing eigenvalues (λ1, λ2, and λ3) and eigenvectors (ε1, ε2, and ε3) used to define an ellipsoid that represents an isosurface of diffusion probability aimed at understanding the microstructural properties of the brain tissue (Huisman, 2010; O'Donnell & Westin, 2011; Zhang et al., 2020). Four DTI indices are commonly used to quantify the shape of the tensors in each brain voxel. The fractional anisotropy (FA) is the most widely used anisotropy measure, an index of the amount of diffusion asymmetry within a voxel. When λ1 = λ2 = λ3, the diffusion ellipsoid is a sphere indicating a perfect isotropic diffusion (FA = 0). With progressive diffusion anisotropy, the eigenvalues become more unequal, and FA values became higher. A complementary measure to FA is mean diffusivity (MD) computed as the average of the three eigenvalues of the tensor. Finally, AD and RD could be helpful in determining the diffusivity direction, along the main axis (λ1) or perpendicular to it (average of λ2 and λ3). FA is sensitive to axonal integrity, although many factors are linked with FA changes (i.e., cell death, gliosis, demyelination, increase in extracellular or intracellular liquid content, inflammation, and axonal loss). Therefore, FA is not a specific parameter to define the type of changes (Neeb et al., 2015; O'Donnell & Westin, 2011; Zhang et al., 2020) and is usually paired with MD. High MD indicates increased extracellular spaces because of shrinkage or degeneration of axons and dendritic fibers. Thus, MD is higher in cerebrospinal fluid (CSF) compared to GM and WM, as water molecules can move freely (Narr et al., 2009; Tromp & Scalars, 2016). Finally, AD and RD could be used to detect axon myelination or pathology (Zhang et al., 2020). AD is sensitive to axonal degeneration, which is associated with fiber density and axon intrinsic characteristics (Messina et al., 2015; Neeb et al., 2015). Whereas demyelination, abnormal axonal diameter, or density may influence RD (Messina et al., 2015; Tromp & Scalars, 2016).
According to a recent coordinate-based meta-analysis consisting of both volume and surface GM and DTI studies, there is no clear consensus about brain structural alterations in migraine (Masson et al., 2021a, b). However, several DTI studies showed widespread alterations of the diffusivity metrics, suggesting a multifaceted association between migraine and brain structural connection/organization (Kim et al., 2021). Herein, we aim to systematically review DTI studies and comprehensively discuss microstructural changes in migraine. Moreover, we aim to clarify whether these changes are associated with clinical parameters, including attack duration, frequency, disease duration, and different phases of migraine.
Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009).
Literature search and selection criteria
We performed an online search in PubMed and Scopus databases in January 2022. The search terms included "Diffusion Tensor Imaging OR Diffusion Magnetic Resonance Imaging OR Diffusion-Weighted Imaging OR Fractional Anisotropy OR Diffusivity OR Tractography" AND "Migraine OR Migraine Disorders OR Migraine Headaches OR Migraine with Aura OR Migraine without Aura OR Chronic Migraine OR Episodic Migraine", and the equivalent search terms in each database. Reference lists of the included studies and other relevant studies were also reviewed for eligible studies.
Original studies in English were included if they (1) measured tract-based or region of interest (ROI) diffusion metrics through computational DTI methods and (2) compared microstructural changes in patients with migraine with healthy controls (HCs) or microstructural features between patients with different migraine types (e.g., with or without aura).
We excluded (1) case reports, case series, letters, commentaries, abstracts, review articles, and animal or in vitro studies, (2) studies including patients diagnosed with different neurologic conditions, and (3) interventional studies.
Data selection was performed in concordance with the PRISMA guidelines (Moher et al., 2009). Two authors (RR and MHA) independently assessed the eligibility criteria of the studies. In case of conflicting judgments, a third author’s (MD) opinion was asked.
Data extraction
The extracted data included: (1) demographic features of the samples, including age and sex of patients and HCs, (2) data related to the disease characteristics, including classification of migraine (with or without aura), disease duration, and attack frequency and duration, (3) the characteristics of image acquisition, including field strength and b-value, (4) DTI analysis methodology, (5) the spectrum of data analysis (whole brain or tract-based), (6) key findings, including the alterations of diffusion metrics across brain regions or tracts, and (7) other relevant findings.
Results
Study selection
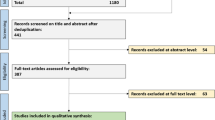
The PRISMA chart for studies selection is depicted in Fig. 1. A total of 646 articles were identified. After removing duplicate records, title and abstract of 409 studies were screened, leading to the exclusion of 361 studies. Of the remaining 48 studies that entered full-text screening, 35 studies were finally included. Thirteen studies were excluded due to the following reasons: network-based DTI analysis (six studies), histogram analysis in patients with WM lesions (three studies), using of imaging modalities other than DTI (two studies), interventional design (one study), and participants with a different neurological condition (one study). Table 1 summarizes the included studies. Reviewed articles included data from 2220 individuals (574 males) consisting of 1253 individuals diagnosed with migraine (295 male patients) by the International Classification of Headache (ICHD) criteria (second and third edition) (Headache Classification Committee of the International Headache, 2013; "Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition," 2018; Olesen & Steiner, 2004), and 967 HCs.
Study characteristics
Data for mean disease duration (years since patients received migraine diagnosis) and attack frequency (the number of migraine headache attacks per month or days) were reported for all articles but Delic et al. (2016), Gomez-Beldarrain et al. (2015), and Kattem Husoy et al. (2019). Mean disease duration ranged from less than three years (Messina et al., 2015) to about 34 years (Schmitz et al., 2008) among the included articles. Several studies reported mean attack duration, defined as the number of hours each attack lasts (Coppola et al., 2014, 2020; Liu et al., 2011; Li et al., 2011; Mettenburg et al., 2012; Yu et al., 2013b; Zhang et al., 2012). Comparing studies which investigated depression and migraine as separate entities and studies focused on concomitant migraine and depression could be helpful in unraveling the distinction between brain diffusivity alterations inherent to these disorders. As decreased AD in WM tracts such as CC, IC and EC was reported in both depressed and non-depressed patients with migraine, axonal loss might be attributable to migraine pathophysiology rather than depressive symptoms, while RD increase and FA reduction, which are presumed markers of demyelination, are observable more frequently in MWOA patients with depressive symptoms (van Velzen et al., 2020; Yu et al., 2013b). Thus, it can be assumed that axonal degeneration and brain atrophy might highlight the adaptive reaction of neurons in response to frequent migraine attacks, while demyelination might represent the main response to depressive symptoms (Li et al., 2011). Future studies should confirm these assumptions.
Correlation between clinical variables and DTI indices in migraine
Some of the studies discussed in the present review assessed the correlation between age and DTI indices with contrasting results. It is worthy of note that participants of the included studies were mainly middle-aged, a period of time when microstructural changes are minimal (Behler et al., 2021). For the aim of understanding age-related microstructural changes in migraine, DTI metrics should be carefully evaluated through longitudinal studies. Similarly, no correlations were reported between the main DTI indices with disease duration, frequency of migraine attacks, and pain intensity. However, preliminary evidence reported a significant positive association between both attack frequency and disease duration with higher MD and lower FA mainly within the CC, thalamic radiations, and CST. These findings, although inconsistent and heterogeneous, might suggest potential deleterious effects of frequent migraine attacks on myelination, which might emerge as a function of the severity of the disease. This might explain the heterogeneity of the findings, although further studies should assess this relationship to unravel the underlying pathophysiological mechanisms influencing brain microstructure by migraine attacks.
Beyond diffusion tensor imaging
Diffusion MRI investigate microstructural structures in vivo in the biologic tissue, detecting early WM microstructural changes. However, to date, DTI indices are non-specific biomarkers for several neurological disorders, including migraine (Alexander et al., 2007). The lack of specificity might depend on some limitations, including subject motion, image resolution, partial volume effect, and crossing WM fibers resulting in potentially biased FA computation (Alexander et al., 2007; Pasternak et al., 2018). Furthermore, DTI technique owns several intrinsic limitations in the detection of GM abnormalities. Indeed, FA can detect water diffusion restriction in anisotropic areas, while GM, consisting of neuronal body, has isotropic properties as diffusion of water molecules is not restricted (Ghazi Sherbaf et al., 2018). These issues might confound the results of migraine studies, masking some potential effects.
Additionally, numerous WM brain structures consist of several complex fiber arrangements (e.g., the CR and the WM adjacent to the cortex), and due to the multiple cross-fibers, DTI indices in these tracts might be suboptimal (Deligianni et al., 2016; Pasternak et al., 2018). Moreover, in neurological disorders, brain microstructure can be affected by the combination of several alterations such as gliosis, inflammation, demyelination, axonal loss and plastic neuronal changes, which are known to affect DTI metrics, making the interpretation of such alterations problematic (Alexander et al., 2007; Pasternak et al., 2018). Moreover, DTI model assumes Gaussian distribution for diffusion within each voxel (Pasternak et al., 2018; Winston, 2015), which is not necessarily the case at the whole brain level (Ghazi Sherbaf et al., 2018). These limitations highlight the necessity to apply novel advanced modalities in migraine, such as Diffusion kurtosis imaging (DKI) and neurite orientation dispersion and density imaging (NODDI). These imaging methods might improve the sensitivity, detecting subtle alterations. DKI is based on non-Gaussian diffusion (Ito et al., 2016), which might improve the detection of diffusion abnormalities in both isotropic (e.g., GM) and anisotropic regions based on the degree of diffusion restriction (Ghazi Sherbaf et al., 2018). DKI consists of three main kurtosis indices, including mean kurtosis (MK), axial kurtosis (AK), and radial kurtosis (RK), representing the structural complexity. Overall, these parameters showed higher sensitivity for detecting crossing fibers compared to DTI and are less prone to the partial volume effect and CSF contamination.
NODDI is a novel model to detect morphology of neurites. Similar to DKI, the model is more suitable for both GM and WM, and less CSF contamination is expected for this modality. Nevertheless, NODDI can be applied to diffusion MRI data typically acquired in clinical setting, which might hasten its extensive application in the clinical research field (Winston, 2015). Other techniques might provide valuable microstructural information in migraine, such as the diffusion ensemble average propagator (EAP), although this methodology requires multi-shell acquisitions (and longer acquisition time), making it less feasible within the clinical practice. This limitation can be overcome by applying novel approaches. Apparent Measures Using Reduced Acquisitions (AMURA), a technique assuming that diffusion anisotropy is approximately independent of the radial direction (Aja-Fernández et al., 2020), could reduce the acquisition time. This approach has been recently applied in migraine patients, showing promising results to detect microstructural changes associated with this disorder (Planchuelo-Gómez et al., 2020). Further studies should evaluate the application of advanced statistical and machine learning techniques aimed at assessing the latent relationships between migraine features and microstructural changes, such as multimodal canonical correlation analysis combined with joint independent component analysis (Planchuelo-Gómez et al., 2021).
Limitations and future direction
Despite its strengths, this systematic review is prone to some limitations. About 20% of the included studies were drawn from similar, or partially overlap**, samples of participants, which might have influenced some results. Moreover, despite our efforts to discuss both significant and non-significant results, there is a tendency towards publication of papers with significant results compared to non-significant ones (i.e., publication bias). Indeed, most of the studies reported significant changes in at least one diffusion parameter, while only nine studies reported completely non-significant results among the investigated DTI parameters. Furthermore, the studies included in this review might be prone to some intrinsic limitations, which should be addressed by future studies. First, the number of patients included in most of these studies was lower than 50, which might limit the generalizability of the conclusion that can be drawn. Additionally, as reported in the present review, imaging time at different phases through migraine cycle might influence the results, due to the fluctuations of DTI parameters in the different phases of migraine. Some possible confounding factors (e.g., positive family history of migraine, severity of pain, presence or absence of aura, types of auras, and anxiety/depression profiles of participants, patient's age and sex, medication intake) might influence DTI findings. Similarly, heterogeneity in the study design, clinical features, and analysis methods might lead to conflicting results, making the interpretation of microstructural alterations in migraine more complex (Forkel et al., 2020). Depression also has an effect on brain microstructure, especially the genu of the CC and the left ALIC (Chen et al., 2016). Therefore, it should be considered for patient selection or classification. Moreover, no study has assessed whether a positive family history of migraine or a genetic predisposition is linked to WM microstructure alterations. Finally, most of the studies were cross-sectional, thus it is impossible to infer a cause-effect relationship between migraine and DTI. Longitudinal studies will be extremely helpful to investigate the development of microstructural abnormalities during disease or treatment.
Conclusion
Despite the great effort to investigate the pathophysiology of migraine, the evidence summarized here suggests that future studies are still necessary to unravel brain diffusion alterations linked with this disorder. Preliminary evidence suggest that microstructural alterations occur during the disease. Reduced microstructural integrity was observed in the thalamus, CC, longitudinal fasciculus, and cingulum in patients with migraine compared to controls. However, the tensor model was unable to find remarkable differences between different migraine subtypes. Notably, changes in DTI indices occur in the interictal phase, which might be interpreted within the habituation deficit theory or as neuronal plasticity mechanisms. Moreover, these results might suggest that frequent stimulation and CSD events lead to release of neurotransmitters and pain generation, and finally, cellular damage, as captured by DTI indices. Indeed, repetitive occurrence of neuronal damage can be associated with disruption of WM microstructure and decreased FA in several brain microstructures. In chronic migraine, the variable equilibrium between neuronal damage, due to repetitive pain stimulations, and plastic neuronal changes occurring as compensatory mechanisms might lead to higher heterogeneous results. DTI assessment and interpretation of structural abnormalities in migraine are still questionable due to the complexity of migraine pathophysiology and DTI limitations. Further longitudinal studies, applying novel advanced modalities are required to fully understating the effects of migraine on brain structural connectivity and its progression.
Availability of data and material
Not applicable.
Abbreviations
- MOH:
-
Medication overuse headache
- MWA:
-
Migraine with aura
- MWoA:
-
Migraine without aura
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- MD:
-
Mean diffusivity
- AD:
-
Axial diffusivity
- RD:
-
Radial diffusivity
- PAG:
-
Periaqueductal gray matter
- GM:
-
Gray matter
- WM:
-
White matter
- ILF:
-
Inferior longitudinal fasciculus
- SLF:
-
Superior longitudinal fasciculus
- UF:
-
Uncinate fasciculus
- CC:
-
Corpus callosum
- CSD:
-
Cortical spreading depression
- PLIC:
-
Posterior limb of internal capsule
- ROI:
-
Region of interest
- ACR:
-
Anterior corona radiata
- PCR:
-
Posterior corona radiata
- PTR:
-
Posterior thalamic radiation
- ATR:
-
Anterior thalamic radiation
- IC:
-
Internal capsule
- EC:
-
External capsule
- SpV:
-
Spinal trigeminal nucleus
- CST:
-
Corticospinal tract
- CFN:
-
Cuneiform nucleus
- SDS:
-
Self-rating depression scale
- CM:
-
Chronic migraine
- HC:
-
Healthy control
References
Aja-Fernández, S., de Luis-García, R., Afzali, M., Molendowska, M., Pieciak, T., & Tristán-Vega, A. (2020). Micro-structure diffusion scalar measures from reduced MRI acquisitions. PLoS One, 15(3), e0229526. https://doi.org/10.1371/journal.pone.0229526
Alexander, A. L., Lee, J. E., Lazar, M., & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics : The Journal of the American Society for Experimental NeuroTherapeutics, 4(3), 316–329. https://doi.org/10.1016/j.nurt.2007.05.011
Behler, A., Kassubek, J., Müller, H.P. (2021). Age-Related Alterations in DTI Metrics in the Human Brain-Consequences for Age Correction. Front Aging Neurosci, 13, 682109. https://doi.org/10.3389/fnagi.2021.682109
Charles, A. C., & Baca, S. M. (2013). Cortical spreading depression and migraine. Nature Reviews Neurology, 9(11), 637–644. https://doi.org/10.1038/nrneurol.2013.192
Chen, G., Hu, X., Li, L., Huang, X., Lui, S., Kuang, W., Ai, H., Bi, F., Gu, Z., & Gong, Q. (2016). Disorganization of white matter architecture in major depressive disorder: a meta-analysis of diffusion tensor imaging with tract-based spatial statistics. Scientific Reports, 6(1), 21825. https://doi.org/10.1038/srep21825
Chong, C. D., & Schwedt, T. J. (2015). Migraine affects white-matter tract integrity: A diffusion-tensor imaging study. Cephalalgia, 35(13), 1162–1171. https://doi.org/10.1177/0333102415573513
Chong, C. D., Peplinski, J., Berisha, V., Ross, K., & Schwedt, T. J. (2019). Differences in fibertract profiles between patients with migraine and those with persistent post-traumatic headache. Cephalalgia, 39(9), 1121–1133. https://doi.org/10.1177/0333102418815650
Cole, J., Chaddock, C. A., Farmer, A. E., Aitchison, K. J., Simmons, A., McGuffin, P., & Fu, C. H. (2012). White matter abnormalities and illness severity in major depressive disorder. British Journal of Psychiatry, 201(1), 33–39. https://doi.org/10.1192/bjp.bp.111.100594
Coppola, G., Vandenheede, M., Di Clemente, L., Ambrosini, A., Fumal, A., De Pasqua, V., & Schoenen, J. (2005). Somatosensory evoked high-frequency oscillations reflecting thalamo-cortical activity are decreased in migraine patients between attacks. Brain, 128(Pt 1), 98–103. https://doi.org/10.1093/brain/awh334
Coppola, G., Currà, A., Di Lorenzo, C., Parisi, V., Gorini, M., Sava, S. L., Schoenen, J., & Pierelli, F. (2010). Abnormal cortical responses to somatosensory stimulation in medication-overuse headache. BMC Neurology, 10, 126. https://doi.org/10.1186/1471-2377-10-126
Coppola, G., Tinelli, E., Lepre, C., Iacovelli, E., Di Lorenzo, C., Di Lorenzo, G., Serrao, M., Pauri, F., Fiermonte, G., Bianco, F., & Pierelli, F. (2014). Dynamic changes in thalamic microstructure of migraine without aura patients: a diffusion tensor magnetic resonance imaging study. European Journal of Neurology, 21(2), 287-e213. https://doi.org/10.1111/ene.12296
Coppola, G., Di Renzo, A., Tinelli, E., Di Lorenzo, C., Di Lorenzo, G., Parisi, V., Serrao, M., Schoenen, J., & Pierelli, F. (2016a). Thalamo-cortical network activity during spontaneous migraine attacks. Neurology, 87(20), 2154–2160. https://doi.org/10.1212/wnl.0000000000003327
Coppola, G., Di Renzo, A., Tinelli, E., Lepre, C., Di Lorenzo, C., Di Lorenzo, G., Scapeccia, M., Parisi, V., Serrao, M., Colonnese, C., Schoenen, J., & Pierelli, F. (2016b). Thalamo-cortical network activity between migraine attacks: Insights from MRI-based microstructural and functional resting-state network correlation analysis. The Journal of Headache and Pain, 17(1), 100. https://doi.org/10.1186/s10194-016-0693-y
Coppola, G., Di Renzo, A., Tinelli, E., Petolicchio, B., Di Lorenzo, C., Parisi, V., Serrao, M., Calistri, V., Tardioli, S., Cartocci, G., Caramia, F., Di Piero, V., & Pierelli, F. (2020). Patients with chronic migraine without history of medication overuse are characterized by a peculiar white matter fiber bundle profile. The Journal of Headache and Pain, 21(1), 92. https://doi.org/10.1186/s10194-020-01159-6
Cucchiara, B., Datta, R., Aguirre, G. K., Idoko, K. E., & Detre, J. (2015). Measurement of visual sensitivity in migraine: Validation of two scales and correlation with visual cortex activation. Cephalalgia, 35(7), 585–592. https://doi.org/10.1177/0333102414547782
Cutrer, F. M., Bajwa, Z., & Sabahat, A. (2012). Pathophysiology, clinical manifestations, and diagnosis of migraine in adults. Up To Date.[Online].
DaSilva, A. F., Granziera, C., Tuch, D. S., Snyder, J., Vincent, M., & Hadjikhani, N. (2007). Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport, 18(4), 301–305. https://doi.org/10.1097/WNR.0b013e32801776bb
Datta, R., Aguirre, G. K., Hu, S., Detre, J. A., & Cucchiara, B. (2013). Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia, 33(6), 365–374. https://doi.org/10.1177/0333102412474503
de Tommaso, M., Lo Sito, L., Di Fruscolo, O., Sardaro, M., PiaPrudenzano, M., Lamberti, P., & Livrea, P. (2005). Lack of habituation of nociceptive evoked responses and pain sensitivity during migraine attack. Clinical Neurophysiology, 116(6), 1254–1264. https://doi.org/10.1016/j.clinph.2005.02.018
Delic, J., Alhilali, L. M., Hughes, M. A., Gumus, S., & Fakhran, S. (2016). White matter injuries in mild traumatic brain injury and posttraumatic migraines: Diffusion entropy analysis. Radiology, 279(3), 859–866. https://doi.org/10.1148/radiol.2015151388
Deligianni, F., Carmichael, D. W., Zhang, G. H., Clark, C. A., & Clayden, J. D. (2016). NODDI and tensor-based microstructural indices as predictors of functional connectivity. PLoS One, 11(4), e0153404. https://doi.org/10.1371/journal.pone.0153404
Dodick, D. W. (2018). Migraine. Lancet, 391(10127), 1315–1330. https://doi.org/10.1016/s0140-6736(18)30478-1
Faragó, P., Tuka, B., Tóth, E., Szabó, N., Király, A., Csete, G., Szok, D., Tajti, J., Párdutz, Á., Vécsei, L., & Kincses, Z. T. (2017). Interictal brain activity differs in migraine with and without aura: Resting state fMRI study. The Journal of Headache and Pain, 18(1), 8. https://doi.org/10.1186/s10194-016-0716-8
Forkel, S., Friedrich, P., Thiebaut de Schotten, M., & Howells, H. (2020). White matter variability, cognition, and disorders: a systematic review. https://doi.org/10.1101/2020.04.22.20075127
Gasparini, C. F., Sutherland, H. G., & Griffiths, L. R. (2013). Studies on the pathophysiology and genetic basis of migraine. Current Genomics, 14(5), 300–315. https://doi.org/10.2174/13892029113149990007
Ghazi Sherbaf, F., Same, K., Ashraf-Ganjouei, A., Aarabi, M.H. (2018). Altered white matter microstructure associated with mild and moderate depressive symptoms in young adults, a diffusion tensor imaging study. Neuroreport, 29(8), 685-689. doi: 10.1097/WNR.0000000000001017.
Gomez-Beldarrain, M., Oroz, I., Zapirain, B. G., Ruanova, B. F., Fernandez, Y. G., Cabrera, A., Anton-Ladislao, A., Aguirre-Larracoechea, U., & Garcia-Monco, J. C. (2015). Right fronto-insular white matter tracts link cognitive reserve and pain in migraine patients. The Journal of Headache and Pain, 17, 4. https://doi.org/10.1186/s10194-016-0593-1
Granziera, C., DaSilva, A. F., Snyder, J., Tuch, D. S., & Hadjikhani, N. (2006). Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Medicine, 3(10), e402. https://doi.org/10.1371/journal.pmed.0030402
Gursoy-Ozdemir, Y., Qiu, J., Matsuoka, N., Bolay, H., Bermpohl, D., **, H., Wang, X., Rosenberg, G. A., Lo, E. H., & Moskowitz, M. A. (2004). Cortical spreading depression activates and upregulates MMP-9. Journal of Clinical Investigation, 113(10), 1447–1455. https://doi.org/10.1172/jci21227
Headache Classification Committee of the International Headache Society (IHS). (2018). The international classification of headache disorders, 3rd edition. Cephalalgia, 38(1), 1–211. https://doi.org/10.1177/0333102417738202
Headache Classification Committee of the International Headache, S. (2013). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia, 33(9), 629–808. https://doi.org/10.1177/0333102413485658
Huisman, T. A. (2010). Diffusion-weighted and diffusion tensor imaging of the brain, made easy. Cancer Imaging, 10 Spec no A(1a), S163-171. https://doi.org/10.1102/1470-7330.2010.9023
Ito, K., Kudo, M., Sasaki, M., Saito, A., Yamashita, F., Harada, T., Yokosawa, S., Uwano, I., Kameda, H., & Terayama, Y. (2016). Detection of changes in the periaqueductal gray matter of patients with episodic migraine using quantitative diffusion kurtosis imaging: preliminary findings. Neuroradiology, 58(2), 115–120. https://doi.org/10.1007/s00234-015-1603-8
Jones, E. G. (2002). Thalamic circuitry and thalamocortical synchrony. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 357(1428), 1659–1673. https://doi.org/10.1098/rstb.2002.1168
Judit, A., Sándor, P. S., & Schoenen, J. (2000). Habituation of visual and intensity dependence of auditory evoked cortical potentials tends to normalize just before and during the migraine attack. Cephalalgia, 20(8), 714–719. https://doi.org/10.1111/j.1468-2982.2000.00122.x
Kara, B., KiyatAtamer, A., Onat, L., Ulusoy, L., Mutlu, A., & Sirvanci, M. (2013). DTI findings during spontaneous migraine attacks. Clinical Neuroradiology, 23(1), 31–36. https://doi.org/10.1007/s00062-012-0165-y
Karatas, H., Erdener, S. E., Gursoy-Ozdemir, Y., Lule, S., Eren-Kocak, E., Sen, Z. D., & Dalkara, T. (2013). Spreading depression triggers headache by activating neuronal Panx1 channels. Science, 339(6123), 1092–1095. https://doi.org/10.1126/science.1231897
Katsarava, Z., Buse, D. C., Manack, A. N., & Lipton, R. B. (2012). Defining the differences between episodic migraine and chronic migraine. Current Pain and Headache Reports, 16(1), 86–92. https://doi.org/10.1007/s11916-011-0233-z
Kattem Husoy, A., Eikenes, L., Haberg, A. K., Hagen, K., & Stovner, L. J. (2019). Diffusion tensor imaging in middle-aged headache sufferers in the general population: a cross-sectional population-based imaging study in the Nord-Trondelag health study (HUNT-MRI). The Journal of Headache and Pain, 20(1), 78. https://doi.org/10.1186/s10194-019-1028-6
Kim, S.-K., Nikolova, S., & Schwedt, T. J. (2021). Structural aberrations of the brain associated with migraine: A narrative review. Headache: The Journal of Head and Face Pain, 61(8), 1159–1179. https://doi.org/10.1111/head.14189
Kincses, Z. T., Veréb, D., Faragó, P., Tóth, E., Kocsis, K., Kincses, B., Király, A., Bozsik, B., Párdutz, Á., Szok, D., Tajti, J., Vécsei, L., Tuka, B., & Szabó, N. (2019). Are Migraine With and Without Aura Really Different Entities? Frontiers in Neurology, 10, 982. https://doi.org/10.3389/fneur.2019.00982
Korgaonkar, M. S., Grieve, S. M., Koslow, S. H., Gabrieli, J. D. E., Gordon, E., & Williams, L. M. (2011). Loss of white matter integrity in major depressive disorder: Evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Human Brain Map**, 32(12), 2161–2171. https://doi.org/10.1002/hbm.21178
Li, X. L., Fang, Y. N., Gao, Q. C., Lin, E. J., Hu, S. H., Ren, L., Ding, M. H., & Luo, B. N. (2011). A diffusion tensor magnetic resonance imaging study of corpus callosum from adult patients with migraine complicated with depressive/anxious disorder. Headache, 51(2), 237–245. https://doi.org/10.1111/j.1526-4610.2010.01774.x
Li, K., Liu, L., Yin, Q., Dun, W., Xu, X., Liu, J., & Zhang, M. (2017). Abnormal rich club organization and impaired correlation between structural and functional connectivity in migraine sufferers. Brain Imaging and Behavior, 11(2), 526–540. https://doi.org/10.1007/s11682-016-9533-6
Liu, J., Lan, L., Li, G., Yan, X., Nan, J., **ong, S., Yin, Q., von Deneen, K. M., Gong, Q., Liang, F., Qin, W., & Tian, J. (2013). Migraine-related gray matter and white matter changes at a 1-year follow-up evaluation. The Journal of Pain, 14(12), 1703–1708. https://doi.org/10.1016/j.jpain.2013.08.013
Mainero, C., Boshyan, J., & Hadjikhani, N. (2011). Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Annals of Neurology, 70(5), 838–845. https://doi.org/10.1002/ana.22537
Maniyar, F. H., Sprenger, T., Monteith, T., Schankin, C., & Goadsby, P. J. (2014). Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain, 137(Pt 1), 232–241. https://doi.org/10.1093/brain/awt320
Marciszewski, K. K., Meylakh, N., Di Pietro, F., Macefield, V. G., Macey, P. M., & Henderson, L. A. (2018). Altered brainstem anatomy in migraine. Cephalalgia, 38(3), 476–486. https://doi.org/10.1177/0333102417694884
Marciszewski, KK., Meylakh, N., Di Pietro, F., Macefield, VG., Macey, PM., Henderson, LA. (2019). Fluctuating regional brainstem diffusion imaging measures of microstructure across the migraine cycle. eNeuro, 6(4). https://doi.org/10.1523/eneuro.0005-19.2019
Masson, R., Demarquay, G., Meunier, D., Lévêque, Y., Hannoun, S., Bidet-Caulet, A., & Caclin, A. (2021b). Is Migraine Associated to Brain Anatomical Alterations? New Data and Coordinate-Based Meta-analysis. Brain Topography, 34(3), 384–401. https://doi.org/10.1007/s10548-021-00824-6
Masson, R., Demarquay, G., Meunier, D., Lévêque, Y., Hannoun, S., Bidet-Caulet, A., & Caclin, A. (2021a). Is migraine associated to brain anatomical alterations? New data and coordinate-based meta-analysis. medRxiv, 2020.2002.2018.20024554. https://doi.org/10.1101/2020.02.18.20024554
Messina, R., Rocca, M. A., Colombo, B., Pagani, E., Falini, A., Comi, G., & Filippi, M. (2015). White matter microstructure abnormalities in pediatric migraine patients. Cephalalgia, 35(14), 1278–1286. https://doi.org/10.1177/0333102415578428
Mettenburg, J. M., Benzinger, T. L., Shimony, J. S., Snyder, A. Z., & Sheline, Y. I. (2012). Diminished performance on neuropsychological testing in late life depression is correlated with microstructural white matter abnormalities. Neuroimage, 60(4), 2182–2190. https://doi.org/10.1016/j.neuroimage.2012.02.044
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med, 6(7), e1000097. https://doi.org/10.1371/journal.pmed.1000097
Moskowitz, M. A., Nozaki, K., & Kraig, R. P. (1993). Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. The Journal of Neuroscience, 13(3), 1167–1177
Moulton, E. A., Burstein, R., Tully, S., Hargreaves, R., Becerra, L., & Borsook, D. (2008). Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One, 3(11), e3799. https://doi.org/10.1371/journal.pone.0003799
Narr, K. L., Hageman, N., Woods, R. P., Hamilton, L. S., Clark, K., Phillips, O., Shattuck, D. W., Asarnow, R. F., Toga, A. W., & Nuechterlein, K. H. (2009). Mean diffusivity: a biomarker for CSF-related disease and genetic liability effects in schizophrenia. Psychiatry Research, 171(1), 20–32. https://doi.org/10.1016/j.pscychresns.2008.03.008
Neeb, L., Bastian, K., Villringer, K., Gits, H. C., Israel, H., Reuter, U., & Fiebach, J. B. (2015). No microstructural white matter alterations in chronic and episodic migraineurs: a case-control diffusion tensor magnetic resonance imaging study. Headache, 55(2), 241–251. https://doi.org/10.1111/head.12496
Noseda, R., Jakubowski, M., Kainz, V., Borsook, D., & Burstein, R. (2011). Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. Journal of Neuroscience, 31(40), 14204–14217. https://doi.org/10.1523/jneurosci.3285-11.2011
O’Donnell, L. J., & Westin, C. F. (2011). An introduction to diffusion tensor image analysis. Neurosurgery Clinics of North America, 22(2), 185–196, viii. https://doi.org/10.1016/j.nec.2010.12.004
Olesen, J., & Steiner, T. J. (2004). The international classification of headache disorders, 2nd edn (ICDH-II). Journal of Neurology, Neurosurgery & Psychiatry, 75(6), 808. https://doi.org/10.1136/jnnp.2003.031286
Pasternak, O., Kelly, S., Sydnor, V. J., & Shenton, M. E. (2018). Nov 15). Advances in microstructural diffusion neuroimaging for psychiatric disorders. NeuroImage, 182, 259–282. https://doi.org/10.1016/j.neuroimage.2018.04.051
Petrušić, I., Daković, M., Kačar, K., Mićić, O., & Zidverc-Trajković, J. (2018). Migraine with aura and white matter tract changes. Acta Neurologica Belgica, 118(3), 485–491. https://doi.org/10.1007/s13760-018-0984-y
Planchuelo-Gómez, Á., García-Azorín, D., Guerrero, Á. L., Aja-Fernández, S., Rodríguez, M., & de Luis-García, R. (2020). White matter changes in chronic and episodic migraine: a diffusion tensor imaging study. The Journal of Headache and Pain, 21(1), 1. https://doi.org/10.1186/s10194-019-1071-3
Planchuelo-Gómez, Á., García-Azorín, D., Guerrero, Á. L., Aja-Fernández, S., Rodríguez, M., & de Luis-García, R. (2021). Multimodal fusion analysis of structural connectivity and gray matter morphology in migraine. Human Brain Map**, 42(4), 908–921. https://doi.org/10.1002/hbm.25267
Planchuelo-Gomez, A., & Garcia-Azorin, D. (2019). Structural connectivity alterations in chronic and episodic migraine: A diffusion magnetic resonance imaging connectomics study. 333102419885392. https://doi.org/10.1177/0333102419885392
Qin, Z., He, X. W., Zhang, J., Xu, S., Li, G. F., Su, J., Shi, Y. H., Ban, S., Hu, Y., Liu, Y. S., Zhuang, M. T., Zhao, R., Shen, X. L., Li, J., Liu, J. R., & Du, X. (2019, Sep 2). Structural changes of cerebellum and brainstem in migraine without aura. 20(1), 93. https://doi.org/10.1186/s10194-019-1045-5
Rocca, M. A., Pagani, E., Colombo, B., Tortorella, P., Falini, A., Comi, G., & Filippi, M. (2008). Selective diffusion changes of the visual pathways in patients with migraine: a 3-T tractography study. Cephalalgia, 28(10), 1061–1068. https://doi.org/10.1111/j.1468-2982.2008.01655.x
Rotarska-Jagiela, A., Schönmeyer, R., Oertel, V., Haenschel, C., Vogeley, K., & Linden, D. E. (2008). The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage, 39(4), 1522–1532. https://doi.org/10.1016/j.neuroimage.2007.10.063
Russo, A., Silvestro, M., Trojsi, F., Bisecco, A., De Micco, R., Caiazzo, G., Di Nardo, F., Esposito, F., Tessitore, A., & Tedeschi, G. (2020). Cognitive networks disarrangement in patients with migraine predicts cutaneous allodynia. Headache, 60(7), 1228–1243. https://doi.org/10.1111/head.13860
Schmitz, N., Admiraal-Behloul, F., Arkink, E. B., Kruit, M. C., Schoonman, G. G., Ferrari, M. D., & van Buchem, M. A. (2008). Attack frequency and disease duration as indicators for brain damage in migraine. Headache, 48(7), 1044–1055. https://doi.org/10.1111/j.1526-4610.2008.01133.x
Sevenich, L. (2018). Brain-resident microglia and blood-borne macrophages orchestrate central nervous system inflammation in neurodegenerative disorders and brain cancer. Frontiers in Immunology, 9, 697. https://doi.org/10.3389/fimmu.2018.00697
Shibata, Y., Ishiyama, S., & Matsushita, A. (2018). White matter diffusion abnormalities in migraine and medication overuse headache: A 1.5-T tract-based spatial statistics study. Clinical Neurology and Neurosurgery, 174, 167–173. https://doi.org/10.1016/j.clineuro.2018.09.022
Sporns, O., Tononi, G., & Kötter, R. (2005). The human connectome: A structural description of the human brain. PLOS Computational Biology, 1(4), e42. https://doi.org/10.1371/journal.pcbi.0010042
Stankewitz, A., Schulz, E., & May, A. (2013). Neuronal correlates of impaired habituation in response to repeated trigemino-nociceptive but not to olfactory input in migraineurs: an fMRI study. Cephalalgia, 33(4), 256–265. https://doi.org/10.1177/0333102412470215
Szabo, N., Kincses, Z. T., Pardutz, A., Tajti, J., Szok, D., Tuka, B., Kiraly, A., Babos, M., Voros, E., Bomboi, G., Orzi, F., & Vecsei, L. (2012). White matter microstructural alterations in migraine: a diffusion-weighted MRI study. Pain, 153(3), 651–656. https://doi.org/10.1016/j.pain.2011.11.029
Szabo, N., Farago, P., Kiraly, A., Vereb, D., Csete, G., Toth, E., Kocsis, K., Kincses, B., Tuka, B., Pardutz, A., Szok, D., Tajti, J., Vecsei, L., & Kincses, Z. T. (2018). Evidence for plastic processes in migraine with aura: A diffusion weighted MRI study. Frontiers in Neuroanatomy, 11, 138. https://doi.org/10.3389/fnana.2017.00138
Tantik Pak, A., NacarDogan, S., & Sengul, Y. (2021). Evaluation of structural changes in orbitofrontal cortex in relation to medication overuse in migraine patients: a diffusion tensor imaging study. Arquivos de Neuro-Psiquiatria, 79(6), 483–488. https://doi.org/10.1590/0004-282x-anp-2020-0360
Tedeschi, G., Russo, A., Conte, F., Corbo, D., Caiazzo, G., Giordano, A., Conforti, R., Esposito, F., & Tessitore, A. (2016). Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia, 36(2), 139–147. https://doi.org/10.1177/0333102415584360
Tessitore, A., Russo, A., Conte, F., Giordano, A., De Stefano, M., Lavorgna, L., Corbo, D., Caiazzo, G., Esposito, F., & Tedeschi, G. (2015). Abnormal connectivity within executive resting-state network in migraine with aura. Headache, 55(6), 794–805. https://doi.org/10.1111/head.12587
Tromp, D., & Scalars, D. (2016). How do they relate to brain structure. The Winnower, 3, e146119.
van Velzen, L. S., Kelly, S., Isaev, D., Aleman, A., Aftanas, L. I., Bauer, J., Baune, B. T., Brak, I. V., Carballedo, A., Connolly, C. G., Couvy-Duchesne, B., Cullen, K. R., Danilenko, K. V., Dannlowski, U., Enneking, V., Filimonova, E., Förster, K., Frodl, T., Gotlib, I. H., … Schmaal, L. (2020). White matter disturbances in major depressive disorder: A coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Molecular Psychiatry, 25(7), 1511–1525. https://doi.org/10.1038/s41380-019-0477-2
Welch, K. M. (2005). Brain hyperexcitability: the basis for antiepileptic drugs in migraine prevention. Headache, 45 Suppl 1, S25-32. https://doi.org/10.1111/j.1526-4610.2005.4501008.x
Winston, G. P. (2015). The potential role of novel diffusion imaging techniques in the understanding and treatment of epilepsy. Quantitative Imaging in Medicine and Surgery, 5(2), 279–287. https://doi.org/10.3978/j.issn.2223-4292.2015.02.03
Yeh, F. C., Panesar, S., Fernandes, D., Meola, A., Yoshino, M., Fernandez-Miranda, J. C., Vettel, J. M., & Verstynen, T. (2018). Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage, 178, 57–68. https://doi.org/10.1016/j.neuroimage.2018.05.027
Yu, D., Yuan, K., Qin, W., Zhao, L., Dong, M., Liu, P., Yang, X., Liu, J., Sun, J., Zhou, G., von Deneen, K. M., & Tian, J. (2013a). Axonal loss of white matter in migraine without aura: a tract-based spatial statistics study. Cephalalgia, 33(1), 34–42. https://doi.org/10.1177/0333102412466964
Yu, D., Yuan, K., Zhao, L., Dong, M., Liu, P., Yang, X., Liu, J., Sun, J., Zhou, G., Xue, T., Zhao, L., Cheng, P., Dong, T., von Deneen, K. M., Qin, W., & Tian, J. (2013b). White matter integrity affected by depressive symptoms in migraine without aura: a tract-based spatial statistics study. NMR in Biomedicine, 26(9), 1103–1112. https://doi.org/10.1002/nbm.2924
Yuan, K., Qin, W., Liu, P., Zhao, L., Yu, D., Zhao, L., Dong, M., Liu, J., Yang, X., von Deneen, K. M., Liang, F., & Tian, J. (2012). Reduced fractional anisotropy of corpus callosum modulates inter-hemispheric resting state functional connectivity in migraine patients without aura. PLoS One, 7(9), e45476. https://doi.org/10.1371/journal.pone.0045476
Zhang, Y., Zhang, J., Oishi, K., Faria, A. V., Jiang, H., Li, X., Akhter, K., Rosa-Neto, P., Pike, G. B., Evans, A., Toga, A. W., Woods, R., Mazziotta, J. C., Miller, M. I., van Zijl, P. C., & Mori, S. (2010). Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage, 52(4), 1289–1301. https://doi.org/10.1016/j.neuroimage.2010.05.049
Zhang, A., Leow, A., Ajilore, O., Lamar, M., Yang, S., Joseph, J., Medina, J., Zhan, L., & Kumar, A. (2012). Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology, 37(4), 959–967. https://doi.org/10.1038/npp.2011.279
Zhang, J., Wu, Y. L., Su, J., Yao, Q., Wang, M., Li, G. F., Zhao, R., Shi, Y. H., Zhao, Y., Zhang, Q., Lu, H., Xu, S., Qin, Z., Cui, G. H., Li, J., Liu, J. R., & Du, X. (2017). Assessment of gray and white matter structural alterations in migraineurs without aura. The Journal of Headache and Pain, 18(1), 74. https://doi.org/10.1186/s10194-017-0783-5
Zhang, Y., Vakhtin, A. A., Jennings, J. S., & Massaband, P. (2020). Diffusion tensor tractography of brainstem fibers and its application in pain. 15(2), e0213952. https://doi.org/10.1371/journal.pone.0213952
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
R.R., M.D., and M.H.A. screened the literature; R.R. and F.A. extracted data from studies; R.R., M.D., and G.C. drafted the manuscript; R.R., M.D., M.H.A., and L.P. supervised the study; all authors read the final manuscript and revised the manuscript.
Corresponding author
Ethics declarations
Declarations
Not applicable.
Ethical approval and consent to participate
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors and does not contain any part with the requirement of informed consent for participants.
Consent for publication
All the authors agreed to publication.
Conflicts of interest
Authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahimi, R., Dolatshahi, M., Abbasi-Feijani, F. et al. Microstructural white matter alterations associated with migraine headaches: a systematic review of diffusion tensor imaging studies. Brain Imaging and Behavior 16, 2375–2401 (2022). https://doi.org/10.1007/s11682-022-00690-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-022-00690-1