Abstract
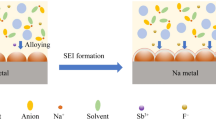
In recent years, rechargeable solid-state sodium metal batteries have become promising energy storage devices with potentially high energy density and low cost. However, eliminating dendrite growth and achieving a stable electrode–electrolyte interface are primary challenges to be addressed. A type of “quasi-liquid” sodium-potassium alloy (Na-K) anode containing only 2 wt% potassium (NaK-2) together with a gel polymer electrolyte exhibits excellent electrochemical performance and can be cycled reversibly for 250 h at 0.2 mA cm−2. In addition, it was found that the formation of the inorganic compound K2O phase during cycling may strengthen the electrode–electrolyte interface, which is helpful for a uniform electrode–electrolyte interface and inhibits dendrite growth, thus improving the cycle performance of the electrode. The quasi-liquid NaK-2 anode paired with Na3V2(PO4)3 exhibits excellent electrochemical performance in the whole battery compared with the pristine metal sodium anode. Our work shows that NaK-2 as an anode material may promote the application of solid-state Na metal batteries.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the ever-growing demand for high-energy density storage systems at a reasonable cost has led researchers to explore new rechargeable secondary batteries such as solid-state sodium metal batteries.1,2,3,4,5 Metallic sodium anodes possess several desirable properties, including a high specific capacity (1160 mAh g−1) and low electrode potential (− 2.714 V compared to the standard hydrogen potential),6,7 has attracted increasing attention in both academic and industrial fields.8,9 Furthermore, its high abundance and low price have made it a research priority and a potential direction for industrialization.28,29,30 Full cell by paring a NaK-2 anode with an Na3V2(PO4)3 cathode operated stably with notable performances at 0.2 C over 300 cycles, superior to its counterpart using a conventional Na metal anode
Experimental
Preparation of Na-K Electrode
The Na-K alloy was prepared by mixing Na and K ingots in different weight ratios, followed by repeated press-rolling using a roller. The Na-K was eventually shaped into a sheet with a thickness of ~ 1 mm. For Na anodes, they were directly press-rolled into sheets of ~ 1 mm thickness. All the metal or alloy sheets were cut into circular disks 10 mm in diameter for cell fabrication.
Preparation of Gel Polymer Electrolyte
The poly-(ethylene glycol) diacrylate (PEGDA) and liquid electrolyte (1 M NaClO4 in EC:DMC = 1:1 in volume with 5% FEC) were uniformly mixed with a mass ratio of 15:85. Then, 2,2’-azobis(2-methylpropionitrile) as a solidified initiator was added to the solution with the content of 1 wt% of PEGDA.
Cathode Preparation
The cathode components of Na3V2(PO4)3, carbon black (Sigma Aldrich), and polyvinylidene fluoride (Sigma Aldrich) (80:10:10 in mass) were mixed and added to 1-methyl-2-pyrrolidinone (NMP, AR, ≥ 99.0%), then stirred evenly and coated onto aluminum foil. Eventually, the NMP was removed in an 80 °C vacuum oven.
Characterization of the Materials
X-ray diffraction (XRD) patterns of the Na-Ks were obtained by Cu Kα radiation (λ = 1.54178 Å) (D/MAX 2550/PC; RIGAKU). The surface morphologies of the samples were characterized with a field-emission scanning electron microscope (S4800; Hitachi), with energy dispersive spectroscopy (EDS) equipment, and XPS was carried out.
Electrochemical Measurements
The ionic conductivity of the gel electrolyte was calculated by:
where L is the thickness of the electrolyte membrane, A is the electrode area, and R represents the impedance of the symmetrical stainless blocking cells. The electrochemical tests were evaluated by assembling CR2025 coin-type solid-state cells. The cellular cellulose membrane was applied as the separator. The repeated sodium strip**/plating behavior used to evaluate the interface stability during cycling was carried out at a current density of 0.2 mA cm−2 with symmetric cells. The galvanostatic charge–discharge performance of full cells at different rates were conducted between 2.2 and 4.0 V (vs. Na+/Na) at room temperature. The galvanostatic charge/discharge curves were tested using the LAND battery system.
The whole assembly process took place in an Ar-filled glove box. The cathode was placed into the positive shell, and then the gel polymer electrolyte was dropped onto it. After that, the separator, sodium foil, foam nickel, and anode shell were stacked in order. Eventually, the coin-type cells were assembled using a tablet press and then solidified at 70 °C for 2 h to obtain the final solid-state cells. Electrochemical impedance spectroscopy (EIS) tests were performed on the Princeton Applied Research electrochemical system between 0.01 and 106 Hz.
Results and Discussion
Previous research works on Na-K were concentrated mainly on the liquid-state alloy with a typical K:Na composition of 66.3:33.7 (w/w) in the K-dominant region. In contrast, our focus is on the quasi-liquid state in the Na-dominant region of the Na-K equilibrium phase diagram, which is a kind of “liquid + αc (K in Na)” phase. Typically, we prepared several Na-K alloys with different ratios of K such as 2, 5, and 10 (denoted as NaK-2, NaK-5, and NaK-10, respectively). Compared to the original solid Na metal (Fig. 1a), no obvious difference in appearance can be observed for the NaK-2 sample (Fig. 1b). It is worth noting that only a small amount of liquid-state alloy can be found on the surface of NaK-2 (as shown in Fig. 1b, red rectangle). This quasi-liquid state of NaK-2 is expected to have processing properties, which can be easily stamped into a desired shape as an electrode for use. When the K content further goes up to 5 wt%, the alloy is characterized as some aggregates of small particles on its surface exhibiting a mossy morphology, suggesting an increase in surface tension (Fig. 1c). Further increasing the K content to 10.0 wt%, the intrinsic morphology of the alloy broke down into a split state of large particles (Fig. 1d). For the latter two samples, they have worse mechanical properties than metallic Na or NaK-2 alloy, and are no longer suitable for subsequent electrode processing.
The crystal structures of the Na-K alloys and the original Na metal were investigated by XRD. The original Na metal shows diffraction peaks at 29.42°, 42.08°, 52.18°, 69.18°, and 84.40° (Fig. 1e), the peaks can be respectively indexed to the (110), (200), (211), (310), and (321) crystal planes of body-centered cubic Na (ICDD, No. 01-071-4506). Interestingly, the NaK-2 sample demonstrates a significant increase in the intensity of the principal diffractions from (110) and (211). However, there is a noticeable decrease in the intensity of the principal diffraction from (321), indicating that the introduction of K into the Na phase modifies the lattice parameters and crystallinity of metallic Na. As the K content increases to 5.0 wt%, the diffraction from Na (321) decreased further (Fig. 1g). When the K content reached 10.0 wt%, the diffractions from the Na (321) crystal planes disappeared (Fig. 1h). These observations suggest that part of the Na in the alloy has become amorphous or quasi-liquid compared to the original Na. It is important to note that the XRD results do not give evidence to support the absence of a liquid state of the Na-K alloy since no lattice exists in the liquid state.3a. During the long cycle with a current density and cycling capacity of 0.2 mA cm−2/0.2 mAh cm−2, the NaK-2 electrode exhibits stable cycling for 250 h, while the Na electrode is short-circuited after 140 h of cycling. The voltage–time curves of different cycling stages are further compared in Fig. 3b, c, and d. In the early and middle stages of the charge–discharge cycles, the polarization voltage of the symmetric cell with NaK-2 as the electrode remained stable at around 130 mV, which is slightly lower than that of the symmetric cell with Na as the electrode at around 190 mV. After 140 h of cycling, the polarization voltage of the symmetric cell with NaK-2 as the electrode remained stable at around 100 mV, even lower than the initial stage, while the symmetric cell with Na as the electrode was short-circuited.
Ex situ SEM observation was conducted for the Na and NaK-2 electrodes before and after cycling to examine their surface morphology. Before cycling, the surface of the sodium metal electrode had a columnar dense structure without dendrite or crack formation, as seen in Fig. 4a. In contrast, Fig. 4c displays the NaK-2 electrode with a moss-like appearance due to the uniform coating of the liquid Na-K alloy on the surface of the sodium substrate. After cycling, a large number of voids and cracks appeared on the surface of the cycled sodium metal anode (Fig. 4b), indicating significant volume expansion and serious sodium dendrite strip**. In contrast, the surface of the cycled NaK-2 electrode (Fig. 4d) remained flat and uniform with almost no void/crack generation. These comparative results demonstrate that NaK-2 can promote the uniformity of deposition and effectively prevent the formation of sodium dendrites.
SEM images of Na electrode before cycling (a) and after cycles (b) in a symmetrical cell. SEM images of bare NaK-2 electrode before cycling (c) and after cycles (d) in symmetrical cell; the electrochemical impedance spectra of (e) Na|Na and (f) NaK-2|NaK-2 symmetric cells before and after cycles (g–i) XPS spectra of bare Na electrode of (g) O 1 s, (h) Na 1 s, and (i) K 2p after cycles in the symmetrical cell; (j–l) XPS spectra of NaK-2 electrode of (j) O 1 s, (k) Na 1 s, and (l) K 2p after cycles in the symmetrical cell.
EIS tests were carried out on the symmetric cells with Na and NaK-2 as the electrodes before and after cycles to verify the uniform stability of the electrolyte–anode interface. As shown in Fig. 4e and f, before cycling, the symmetric cell with NaK-2 as the electrode has a lower interfacial impedance, with a value of ~ 1000 Ω, which is much smaller than the impedance value of ~ 2000 Ω for the comparison sample under the same conditions. This indicates that the Na-K alloy on the surface of NaK-2 enhances the interfacial contact between the electrode and the electrolyte. After 50 cycles, the impedance value of the symmetric cell with NaK-2 as the electrode slightly increased to ~ 625 Ω, while the comparison sample shows an increased interfacial impedance of ~ 2200 Ω. Furthermore, after 100 cycles, the symmetric cell with Na as the electrode was short-circuited due to the growth of sodium dendrites and an unstable electrode–electrolyte interface. In contrast, the interfacial impedance of the symmetric cell with NaK-2 as the anode electrode was stabilized at ~ 720 Ω, indicating that NaK-2 can form a more stable electrode–electrolyte interface, facilitating the rapid transport of sodium ions at the surface of the gel electrolyte. XPS was used to analyze the specific composition of the anodes with the electrolyte after cycling of the symmetric cells. The O 1 s profiles of the electrolyte–anode interface all exhibit typical reaction products of sodium metal with electrolyte, including C-O (531.11 eV) and C = O (533.12 eV). However, the presence of the inorganic component O-metal (529.7 eV) in the O profile of the NaK-2 anode electrode was observed, which is related to the Na-K alloy contained in the anode electrode with electrolyte reaction.31 Additionally, two peaks of K 2p spectra at 292.8 eV (K-O 2p 3/2) and 295.6 eV (K-O 2p ½) can be assigned to the K2O.32 This peak was not visible in the O profile of the Na anode electrode. The K2O acts as an inorganic component that is present in crystalline grains dispersed in the amorphous organic matrix. It may strengthen the electrolyte–NaK-2 interface like aggregates to concrete.28 As a result, it regulates the crystal growth during the cycling, thus reducing dendrite growth and forming an enhanced electrode–electrolyte interface.
To investigate the practical application of NaK-2, full cells were constructed using sodium vanadium phosphate (NVP) as the cathode material. The results presented in Fig. 5a indicate that the full cell with Na as the anode has an initial capacity of 104.6 mAh g−1 at 0.2 C charge–discharge current density, but only 85.6 mAh g−1 was retained after 300 cycles, delivering a capacity retention of 81.8%. In contrast, the full cell with NaK-2 as the anode (shown in Fig. 5b and c) has an initial capacity of approximately 110.4 mAh g−1 , and still has a capacity of 97.7 mAh g−1 after 300 cycles, demonstrating a capacity retention of 88.7%. Moreover, using NaK-2 as the anode results in a full cell with a higher Coulombic efficiency of 99.7%, superior to the full cell with Na as the anode (99.16%). As depicted in Fig. S2, the full cell with NaK-2 not only exhibits a high discharge capacity and Coulombic efficiency but also demonstrates a small polarization voltage. This can be mainly attributed to the formation of a dense and stable anode electrode–electrolyte interface, which effectively prevents side reactions between the sodium metal and the gel electrolyte. Figure 5d illustrates the rate performance of the full cells at different current densities. The average discharge capacity of the full cell with NaK-2 as the anode is 109.8 mAh g−1, 101.4 mAh g−1, 86.2 mAh g−1, and 70.8 mAh g−1 at 0.2 C, 0.5 C, 1 C, and 2 C, respectively. Notably, when the current density returned to 0.2 C, the discharging capacity rebounded to 102.8 mAh g−1, equivalent to 93.6% of the initial capacity. Conversely, the full cell with Na as the anode electrode exhibited a poor rate performance, with the discharge capacity only reaching 89.4% of the initial capacity when the current density decreased to 0.2 C. The enhanced rate performance was attributed to the superior electrode–electrolyte interface.
Conclusions
A quasi-liquid Na-K alloy anode was explored by finely regulating the mass ratio of sodium and potassium. The NaK-2 alloy material not only has good molding processability but also a high dendrite suppression ability of the liquid Na-K alloy. Additionally, due to the high reactivity of potassium in the liquid Na-K alloy, the inorganic component K2O is formed on the electrode surface during activation cycling stage, which can help to construct an ultra-stable electrolyte–anode interface. When matching with a gel electrolyte with high ionic conductivity, the symmetric cell using NaK-2 electrode can be stably cycled for more than 250 h at 0.2 mA cm−2. Furthermore, favored by the enhanced interfacial properties, the NaK-2 anode endowed solid-state batteries with an outstanding performance while coupled with an NVP cathode. The full cell presents an enhanced rate performance and long-term cycling stability with a high capacity retention of 88.7% after 300 cycles.
References
Q.L. Ma and F. Tietz, Solid-state electrolyte materials for sodium batteries: towards practical applications. ChemElectroChem 7, 2693 (2020). https://doi.org/10.1002/celc.202000164.
Y. Yao, Z.Y. Wei, H.Y. Wang, H.J. Huang, Y. Jiang, X.J. Wu, X.Y. Yao, Z.S. Wu, and Y. Yu, Toward high energy density all solid-state sodium batteries with excellent flexibility. Adv. Energy Mater. 10, 1903698 (2020). https://doi.org/10.1002/aenm.201903698.
H.S. Hirsh, Y.X. Li, D.H.S. Tan, M.H. Zhang, E.Y. Zhao, and Y.S. Meng, Sodium-ion batteries paving the way for grid energy storage. Adv. Energy Mater. 10, 2001274 (2020). https://doi.org/10.1002/aenm.202001274.
T. Deng, X. Ji, L. Zou, O. Chiekezi, L. Cao, X. Fan, T.R. Adebisi, H.J. Chang, H. Wang, B. Li, X. Li, C. Wang, D. Reed, J.G. Zhang, V.L. Sprenkle, C. Wang, and X. Lu, Interfacial-engineering-enabled practical low-temperature sodium metal battery. Nat. Nanotechnol. 17, 269 (2022). https://doi.org/10.1038/s41565-021-01036-6.
W. Niu, L. Chen, Y.C. Liu, and L.Z. Fan, All-solid-state sodium batteries enabled by flexible composite electrolytes and plastic-crystal interphase. Chem. Eng. J. 384, 123233 (2020). https://doi.org/10.1016/j.cej.2019.123233.
R. Usiskin, Y.X. Lu, J. Popovic, M. Law, P. Balaya, Y.S. Hu, and J. Maier, Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 6, 1020 (2021). https://doi.org/10.1038/s41578-021-00324-w.
C. Zhao, L. Liu, X. Qi, Y. Lu, F. Wu, J. Zhao, Y. Yu, Y.-S. Hu, and L. Chen, Solid-state sodium batteries. Adv. Energy Mater. 8, 1703012 (2018). https://doi.org/10.1002/aenm.201703012.
X. Yu, L. Xue, J.B. Goodenough, and A. Manthiram, Ambient-temperature all-solid-state sodium batteries with a laminated composite electrolyte. Adv. Funct. Mater. 31, 2002144 (2020). https://doi.org/10.1002/adfm.202002144.
H. Yin, C. Han, Q. Liu, F. Wu, F. Zhang, and Y. Tang, Recent advances and perspectives on the polymer electrolytes for sodium/potassium-ion batteries. Small 17, e2006627 (2021). https://doi.org/10.1002/smll.202006627.
C. Yang, S. **n, L. Mai, and Y. You, Materials design for high-safety sodium-ion battery. Adv. Energy Mater. 11, 2000974 (2021). https://doi.org/10.1002/aenm.202000974.
B. Sun, P. **ong, U. Maitra, D. Langsdorf, K. Yan, C. Wang, J. Janek, D. Schroder, and G. Wang, Design strategies to enable the efficient use of sodium metal anodes in high-energy batteries. Adv. Mater. 32, e1903891 (2020). https://doi.org/10.1002/adma.201903891.
L. He, L. Ruan, W. Yao, C. Cai, Z. Chen, X. Chang, J. Shi, T. Liu, S. Shen, and Z. Yao, Tailoring sodium iron hexacyanoferrate/carbon nanotube arrays with 3D networks for efficient sodium ion storage. J. Electron. Mater. (2023). https://doi.org/10.1007/s11664-023-10337-6.
K. Fan, C. Wei, and J. Feng, Recent progress of MXene-based materials as anodes in sodium-ion batteries. J. Electron. Mater. 52, 847 (2023). https://doi.org/10.1007/s11664-022-10142-7.
J. Lu, Z. Zhang, Y. Zheng, and Y. Gao, In-situ Transmission electron microscopy for sodium-ion batteries. Adv. Mater. (2023). https://doi.org/10.1002/adma.202300359.
G.H. Chen, K. Zhang, Y.R. Liu, L. Ye, Y.S. Gao, W.R. Lin, H.J. Xu, X.R. Wang, Y. Bai, and C. Wu, Flame-retardant gel polymer electrolyte and interface for quasi-solid-state sodium ion batteries. Chem. Eng. J. 401, 126065 (2020). https://doi.org/10.1016/j.cej.2020.126065.
D.A. Rakov, F. Chen, S.A. Ferdousi, H. Li, T. Pathirana, A.N. Simonov, P.C. Howlett, R. Atkin, and M. Forsyth, Engineering high-energy-density sodium battery anodes for improved cycling with superconcentrated ionic-liquid electrolytes. Nat Mater. 19, 1096 (2020). https://doi.org/10.1038/s41563-020-0673-0.
W.C. Zhang, J. Lu, and Z.P. Guo, Challenges and future perspectives on sodium and potassium ion batteries for grid-scale energy storage. Mater. Today 50, 400 (2021). https://doi.org/10.1016/j.mattod.2021.03.015.
T.T. Luo, Q.Q. Zhao, Y.W. Liu, W.D. Meng, Q. Sun, L. Dai, S. Liu, and L. Wang, High-rate and dendrite-free liquid alloy anode for high energy potassium metal batteries. Ecomat (2022). https://doi.org/10.1002/eom2.12203.
Y. Li, H. Wang, W. Yuan, Y. Luo, J. Tu, L. Zhang, and J. Shu, Ion competition and limiting dendrite growth models of hybrid-ion symmetric cell. Energy Storage Mater. 42, 268 (2021). https://doi.org/10.1016/j.ensm.2021.07.035.
L. Xue, H. Gao, W. Zhou, S. **n, K. Park, Y. Li, and J.B. Goodenough, Liquid K-Na alloy anode enables dendrite-free potassium batteries. Adv. Mater. 28, 9608 (2016). https://doi.org/10.1002/adma.201602633.
X.L. Guo, J. Bae, Y. Ding, X. Zhang, and G.H. Yu, Liquid alloy enabled solid-state batteries for conformal electrode–electrolyte interfaces. Adv. Funct. Mater. 31, 2010863 (2021). https://doi.org/10.1002/adfm.202010863.
L. Zhang, X. **a, Y. Zhong, D. **e, S. Liu, X. Wang, and J. Tu, Exploring self-healing liquid Na-K Alloy for dendrite-free electrochemical energy storage. Adv. Mater. 30, e1804011 (2018). https://doi.org/10.1002/adma.201804011.
W. Yuan, T. Ding, P. Mou, Y. Luo, L. Li, Y. Chen, X. Chen, J. Shu, and L. Zhang, semi-solid CNT@NaK anode for potassium metal battery. Adv. Funct. Mater. 33, 2209774 (2022). https://doi.org/10.1002/adfm.202209774.
Y.S. Luo, P.Z. Mou, W.L. Yuan, L.P. Li, Y.Z. Fan, Y. Chen, X.M. Chen, J. Shu, and L.Y. Zhang, Anti-liquid metal permeation separator for stretchable potassium metal batteries. Chem. Eng. J. 452, 139157 (2023). https://doi.org/10.1016/j.cej.2022.139157.
C.L. Wei, L.W. Tan, Y.C. Zhang, Z.R. Wang, B.J. **, S.L. **ong, J.K. Feng, and Y.T. Qian, Review of room-temperature liquid metals for advanced metal anodes in rechargeable batteries. Energy Storage Mater. 50, 473 (2022). https://doi.org/10.1016/j.ensm.2022.05.024.
L.Y. Zhang, Y.Q. Li, S.Z. Zhang, X.L. Wang, X.H. **a, D. **e, C.D. Gu, and J.P. Tu, Non-Newtonian fluid state K-Na alloy for a stretchable energy storage device. Small Methods 3, 1900383 (2019). https://doi.org/10.1002/smtd.201900383.
Z. Tai, Y. Li, Y. Liu, L. Zhao, Y. Ding, Z. Lu, Z. Peng, L. Meng, G. Yu, and L. Liu, Novel quasi-liquid K-Na alloy as a promising dendrite-free anode for rechargeable potassium metal batteries. Adv. Sci. (Weinh.) 8, e2101866 (2021). https://doi.org/10.1002/advs.202101866.
J.Y. Hu, H.W. Wang, S.W. Wang, Y. Lei, L. Qin, X.J. Li, D.Y. Zhai, B.H. Li, and F.Y. Kang, Electrochemical deposition mechanism of sodium and potassium. Energy Storage Mater. 36, 91 (2021). https://doi.org/10.1016/j.ensm.2020.12.017.
Y.Z. Li, W. Huang, Y.B. Li, A. Pei, D.T. Boyle, and Y. Cui, Correlating structure and function of battery interphases at atomic resolution using cryoelectron microscopy. Joule 2, 2167 (2018). https://doi.org/10.1016/j.joule.2018.08.004.
Y. Li, Y. Li, A. Pei, K. Yan, Y. Sun, C.L. Wu, L.M. Joubert, R. Chin, A.L. Koh, Y. Yu, J. Perrino, B. Butz, S. Chu, and Y. Cui, Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506 (2017). https://doi.org/10.1126/science.aam6014.
Y. Lei, D. Han, J. Dong, L. Qin, X. Li, D. Zhai, B. Li, Y. Wu, and F. Kang, Unveiling the influence of electrode/electrolyte interface on the capacity fading for typical graphite-based potassium-ion batteries. Energy Storage Mater. 24, 319 (2020). https://doi.org/10.1016/j.ensm.2019.07.043.
J. Zhang, Y. Li, L. Zhu, X. Wang, and J. Tu, An intercalation compound for high-safe K metal batteries. Energy Storage Mater. 41, 606 (2021). https://doi.org/10.1016/j.ensm.2021.06.038.
Acknowledgments
This work is supported by National Natural Science Foundation of China (Grant. No. U20A20126, 51971201, 52073259) and Key Research and Development Program of Zhejiang Province (2022C01071).
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, K., Chen, D., Yin, C. et al. Suppressing Dendrite Growth with Quasi-liquid Anode in Solid-State Sodium Metal Batteries Enabled by the Design of Na-K Alloying Strategy. J. Electron. Mater. 52, 5352–5361 (2023). https://doi.org/10.1007/s11664-023-10504-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10504-9