Abstract
Solid-state batteries have shown the potential to resolve the safety and durability issues associated with traditional liquid electrolyte-based batteries. This article reviews the current developments of NASICON-type solid electrolytes for Na-ion solid-state batteries. These ceramic-based oxides possess a 3D open-framework structure allowing for the fast diffusion of large sodium ions. To date, the conductivity value as high as ~ 5 mS·cm−1 at 25 °C is reported for these materials, which needs to be further improved. The requirement of high-temperature sintering (> 1200 °C), anisotropic thermal expansion, impurity phase formation, and large interfacial impedance are other challenges of NASICON-type electrolytes. This article summarizes various fundamental aspects governing the sodium-ion conduction in these oxides. Particular emphasis is given to the strategies employed in recent investigations to improve the properties and alleviate the associated issues in designing stable solid-state sodium-ion rechargeable batteries. This will also establish the groundwork for future research in these materials.
Graphical Abstract


(Source: Scopus, Google scholar)


(Reproduced with permission from ref. no. [70])


(Reproduced with permission from ref. no. [72])



(Reproduced with permission from ref. no. [93])


(Reproduced with permission from ref. no. [146])

(Reproduced with permission from ref. no. [70])

(Reproduced with permission from ref. no. [155])

(Reproduced with permission from ref no. [106])

(Reproduced with permission from ref. no. [106])

(Reproduced with permission from ref. no [167].)

(Reproduced with permission from ref. no. [56])

(Reproduced with permission from ref. no. [175])

Similar content being viewed by others
References
Larcher D, Tarascon J-M (2015) Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 7(1):19–29. https://doi.org/10.1038/nchem.2085
Barbir F, Veziroǧlu T, Plass H Jr (1990) Environmental damage due to fossil fuels use. Int J Hydrogen Energy 15(10):739–749. https://doi.org/10.1016/0360-3199(90)90005-J
Gür TM (2018) Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy Environ Sci 11(10):2696–2767. https://doi.org/10.1039/C8EE01419A
Dell RM, Rand DAJ (2001) Energy storage-a key technology for global energy sustainability. J Power Sources 100(1–2):2–17. https://doi.org/10.1016/S0378-7753(01)00894-1
Verma S, Mishra S, Gaur A, Chowdhury S, Mohapatra S, Dwivedi G, Verma P (2021) A comprehensive review on energy storage in hybrid electric vehicle. J Traffic Transp Eng 8(5):621–637. https://doi.org/10.1016/j.jtte.2021.09.001
Sanguesa JA, Torres-Sanz V, Garrido P, Martinez FJ, Marquez-Barja JM (2021) A review on electric vehicles: technologies and challenges. Smart Cities 4(1):372–404. https://doi.org/10.3390/smartcities4010022
Bruce PG, Freunberger SA, Hardwick LJ, Tarascon J-M (2012) Li–O2 and Li–S batteries with high energy storage. Nat Mater 11(1):19–29. https://doi.org/10.1038/nmat3191
Yang Z, Zhang J, Kintner-Meyer MC, Lu X, Choi D, Lemmon JP, Liu J (2011) Electrochemical energy storage for green grid. Chem Rev 111(5):3577–3613. https://doi.org/10.1021/cr100290v
Choi JW, Aurbach D (2016) Promise and reality of post-lithium-ion batteries with high energy densities. Nat Rev Mater 1:16013. https://doi.org/10.1038/natrevmats.2016.13
Armand M, Tarascon J-M (2008) Building better batteries. Nature 451:652–657. https://doi.org/10.1038/451652a
Goodenough JB, Park K-S (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135(4):1167–1176. https://doi.org/10.1021/ja3091438
Sun Y-K, Chen Z, Noh H-J, Lee D-J, Jung H-G, Ren Y, Wang S, Yoon CS, Myung S-T, Amine K (2012) Nanostructured high-energy cathode materials for advanced lithium batteries. Nat Mater 11(11):942–947. https://doi.org/10.1038/nmat3435
Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J (2017) Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33:363–386. https://doi.org/10.1016/j.nanoen.2017.01.028
Zhang W, Nie J, Li F, Wang ZL, Sun C (2018) A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 45:413–419. https://doi.org/10.1016/j.nanoen.2018.01.028
Pender JP, Jha G, Youn DH, Ziegler JM, Andoni I, Choi EJ, Heller A, Dunn BS, Weiss PS, Penner RM, Mullins CB (2020) Electrode degradation in lithium-ion batteries. ACS Nano 14(2):1243–1295. https://doi.org/10.1021/acsnano.9b04365
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18(5):252–264. https://doi.org/10.1016/j.mattod.2014.10.040
Manthiram A (2017) An outlook on lithium-ion battery technology. ACS Cent Sci 3(10):1063–1069. https://doi.org/10.1021/acscentsci.7b00288
U.S. Geological Survey (2020) Mineral commodity summaries 2020, Reston, VA (2020) 204. https://doi.org/10.3133/mcs2020
Hartmann P, Bender CL, Vračar M, Dürr AK, Garsuch A, Janek J, Adelhelm P (2013) A rechargeable room-temperature sodium superoxide (NaO2) battery. Nat Mater 12(3):228–232. https://doi.org/10.1038/nmat3486
Yabuuchi N, Kubota K, Dahbi M, Komaba S (2014) Research development on sodium-ion batteries. Chem Rev 114(23):11636–11682. https://doi.org/10.1021/cr500192f
**ang X, Zhang K, Chen J (2015) Recent advances and prospects of cathode materials for sodium‐ion batteries. Adv Mater 27(36):5343–5364. https://doi.org/10.1002/adma.201501527
Luo W, Shen F, Bommier C, Zhu H, Ji X, Hu L (2016) Na-ion battery anodes: materials and electrochemistry. Acc Chem Res 49(2):231–240. https://doi.org/10.1021/acs.accounts.5b00482
Su H, Jaffer S, Yu H (2016) Transition metal oxides for sodium-ion batteries. Energy Storage Mater 5:116–131. https://doi.org/10.1016/j.ensm.2016.06.005
Hwang J-Y, Myung S-T, Sun Y-K (2017) Sodium-ion batteries: present and future. Chem Soc Rev 46(12):3529–3614. https://doi.org/10.1039/C6CS00776G
Wang Y, Song S, Xu C, Hu N, Molenda J, Lu L (2019) Development of solid-state electrolytes for sodium-ion battery–A short review. Nano Mater Sci 1(2):91–100. https://doi.org/10.1016/j.nanoms.2019.02.007
Yu Y, Che H, Yang X, Deng Y, Li L, Ma Z-F (2020) Non-flammable organic electrolyte for sodium-ion batteries. Electrochem Commun 110:106635. https://doi.org/10.1016/j.elecom.2019.106635
Che H, Chen S, **e Y, Wang H, Amine K, Liao X-Z, Ma Z-F (2017) Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ Sci 10(5):1075–1101. https://doi.org/10.1039/C7EE00524E
Kim JJ, Yoon K, Park I, Kang K (2017) Progress in the Development of Sodium-Ion Solid Electrolytes. Small Methods 1(10):1700219. https://doi.org/10.1002/smtd.201700219
Hou H, Xu Q, Pang Y, Li L, Wang J, Zhang C, Sun C (2017) Efficient storing energy harvested by triboelectric nanogenerators using a safe and durable all‐solid‐state sodium‐ion battery. Adv Sci 4(8):1700072. https://doi.org/10.1002/advs.201700072
Tian H, Liu S, Deng L, Wang L, Dai L (2021) New-type Hf-based NASICON electrolyte for solid-state Na-ion batteries with superior long-cycling stability and rate capability. Energy Storage Mater 39:232–238. https://doi.org/10.1016/j.ensm.2021.04.026
Zhang C, Gamble S, Ainsworth D, Slawin AM, Andreev YG, Bruce PG (2009) Alkali metal crystalline polymer electrolytes. Nat Mater 8(7):580–584. https://doi.org/10.1038/nmat2474
Hayashi A, Noi K, Sakuda A, Tatsumisago M (2012) Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat Commun 3(1):1–5. https://doi.org/10.1038/ncomms1843
Ma Q, Guin M, Naqash S, Tsai C-L, Tietz F, Guillon O (2016) Scandium-substituted Na3Zr2(SiO4)2(PO4) prepared by a solution-assisted solid-state reaction method as sodium-ion conductors. Chem Mater 28(13):4821–4828. https://doi.org/10.1021/acs.chemmater.6b02059
Zhang Z, Zhang Q, Shi J, Chu YS, Yu X, Xu K, Ge M, Yan H, Li W, Gu L (2017) A Self‐Forming Composite Electrolyte for Solid‐State Sodium Battery with Ultralong Cycle Life. Adv Energy Mater 7(4):1601196. https://doi.org/10.1002/aenm.201601196
Duchêne L, Kühnel R-S, Stilp E, Reyes EC, Remhof A, Hagemann H, Battaglia C (2017) A stable 3 V all-solid-state sodium–ion battery based on a closo-borate electrolyte. Energy Environ Sci 10(12):2609–2615. https://doi.org/10.1039/C7EE02420G
Noguchi Y, Kobayashi E, Plashnitsa LS, Okada S, Yamaki J-I (2013) Fabrication and performances of all solid-state symmetric sodium battery based on NASICON-related compounds. Electrochim Acta 101:59–65. https://doi.org/10.1016/j.electacta.2012.11.038
Wei T, Gong Y, Zhao X, Huang K (2014) An All‐Ceramic Solid‐State Rechargeable Na+‐Battery Operated at Intermediate Temperatures. Adv Funct Mater 24(34):5380–5384. https://doi.org/10.1002/adfm.201400773
Liu C, Massé R, Nan X, Cao G (2016) A promising cathode for Li-ion batteries: Li3V2(PO4)3. Energy Storage Mater 4:15–58. https://doi.org/10.1016/j.ensm.2016.02.002
Ramesh A, Tripathi A, Balaya P (2022) Int. J. Appl. Ceram. Technol. 19(2): 913
Ni Q, Bai Y, Wu F, Wu C (2017) Adv. Sci. 4(3):1600275
Zheng F, Kotobuki M, Song S, Lai MO, Lu L (2018) Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sources 389:198–213. https://doi.org/10.1016/j.jpowsour.2018.04.022
Ngai KS, Ramesh S, Ramesh K, Juan JC (2016) A review of polymer electrolytes: fundamental, approaches and applications. Ionics 22(8):1259–12779. https://doi.org/10.1007/s11581-016-1756-4
Golodnitsky D, Strauss E, Peled E, Greenbaum S (2015) On order and disorder in polymer electrolytes. J Electrochem Soc 162(14):A2551–A2566. https://doi.org/10.1149/2.0161514jes
Fergus JW (2012) Ion transport in sodium ion conducting solid electrolytes. Solid State Ionics 227:102–112. https://doi.org/10.1016/j.ssi.2012.09.019
Zhao C, Liu L, Qi X, Lu Y, Wu F, Zhao J, Yu Y, Hu YS, Chen L (2018) Solid‐state sodium batteries. Adv Energy Mater 8(17):1703012. https://doi.org/10.1002/aenm.201703012
Lu Y, Li L, Zhang Q, Niu Z, Chen J (2018) Electrolyte and interface engineering for solid-state sodium batteries. Joule 2(9):1747–1770. https://doi.org/10.1016/j.joule.2018.07.028
Hueso KB, Armand M, Rojo T (2013) High temperature sodium batteries: status, challenges and future trends. Energy Environ Sci 6(3):734–749. https://doi.org/10.1039/C3EE24086J
Rajagopalan R, Chen B, Zhang Z, Wu XL, Du Y, Huang Y, Li B, Zong Y, Wang J, Nam GH (2017) Improved reversibility of Fe3+/Fe4+ redox couple in sodium super ion conductor type Na3Fe2(PO4)3 for sodium‐ion batteries. Adv Mater 29(12):1605694. https://doi.org/10.1002/adma.201605694
Fu L, Xue X, Tang Y, Sun D, **e H, Wang H (2018) Size controlling and surface engineering enable NaTi2(PO4)3/C outstanding sodium storage properties. Electrochim Acta 289:21–28. https://doi.org/10.1016/j.electacta.2018.09.024
Leng H, Huang J, Nie J, Luo J (2018) Cold sintering and ionic conductivities of Na3.256Mg0.128Zr1.872Si2PO12 solid electrolytes. J Power Sources 391:170–179. https://doi.org/10.1016/j.jpowsour.2018.04.067
Anantharamulu N, Rao KK, Rambabu G, Kumar BV, Radha V, Vithal M (2011) A wide-ranging review on Nasicon type materials. J Mater Sci 46(9):2821–2837. https://doi.org/10.1007/s10853-011-5302-5
Kim J-K, Lim YJ, Kim H, Cho G-B, Kim Y (2015) A hybrid solid electrolyte for flexible solid-state sodium batteries. Energy Environ Sci 8(12):3589–3596. https://doi.org/10.1039/C5EE01941A
Zhang Z, Zhang Q, Ren C, Luo F, Ma Q, Hu Y-S, Zhou Z, Li H, Huang X, Chen L (2016) A ceramic/polymer composite solid electrolyte for sodium batteries. J Mater Chem A 4(41):15823–15828. https://doi.org/10.1039/C6TA07590H
Jolley AG, Cohn G, Hitz GT, Wachsman ED (2015) Improving the ionic conductivity of NASICON through aliovalent cation substitution of Na3Zr2Si2PO12. Ionics 21(11):3031–3038. https://doi.org/10.1007/s11581-015-1498-8
Adelhelm P, Hartmann P, Bender CL, Busche M, Eufinger C, Janek J (2015) From lithium to sodium: cell chemistry of room temperature sodium–air and sodium–sulfur batteries. Beilstein J Nanotechnol 6:1016–1055. https://doi.org/10.3762/bjnano.6.105
Zhou W, Li Y, **n S, Goodenough JB (2017) Rechargeable sodium all-solid-state battery. ACS Cent Sci 3(1):52–57. https://doi.org/10.1021/acscentsci.6b00321
Rajagopalan R, Zhang Z, Tang Y, Jia C, Ji X, Wang (2021) Energy Storage Mater. 34:171
Rao YB, Bharathi KK, Patro L (2021) Review on the synthesis and do** strategies in enhancing the Na ion conductivity of Na3Zr2Si2PO12 (NASICON) based solid electrolytes. Solid State Ionics 366:115671
Yang Z, Tang B, **e Z, Zhou Z (2021) NASICON-Type Na3Zr2Si2PO12 Solid-State Electrolytes for Sodium Batteries. ChemElectroChem 8(6):1035–1047. https://doi.org/10.1002/celc.202001527
Hong H-P (1976) Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater Res Bull 11(2):173–182. https://doi.org/10.1016/0025-5408(76)90073-8
Bui KM, Dinh VA, Okada S, Ohno T (2016) Na-ion diffusion in a NASICON-type solid electrolyte: a density functional study. Phys Chem Chem Phys 18(39):27226–27231. https://doi.org/10.1039/C6CP05164B
Aono H, Sugimoto E, Sadaoka Y, Imanaka N, Adachi GY (1989) Ionic Conductivity of the Lithium Titanium Phosphate (Li1 + X M X Ti2 − X (PO 4) 3, M = Al, Sc, Y, and La) Systems. J Electrochem Soc 136(2):590. https://doi.org/10.1149/1.2096693
Khireddine H, Fabry P, Caneiro A, Bochu B (1997) Optimization of NASICON composition for Na+ recognition. Sens Actuators B 40(2):223–230. https://doi.org/10.1016/S0925-4005(97)80266-3
Hayashi A, Masuzawa N, Yubuchi S, Tsuji F, Hotehama C, Sakuda A, Tatsumisago M (2019) A sodium-ion sulfide solid electrolyte with unprecedented conductivity at room temperature. Nat Commun 10(1):5266. https://doi.org/10.1038/s41467-019-13178-2
Ouyang B, Wang J, He T, Bartel CJ, Huo H, Wang Y, Lacivita V, Kim H, Ceder G (2021) Synthetic accessibility and stability rules of NASICONs. Nat Commun 12(1):5752. https://doi.org/10.1038/s41467-021-26006-3
Jolley AG, Taylor DD, Schreiber NJ, Wachsman ED (2015) Structural Investigation of Monoclinic‐Rhombohedral Phase Transition in Na3Zr2Si2PO12 and Doped NASICON. J Am Ceram Soc 98(9):2902–2907. https://doi.org/10.1111/jace.13692
Deng Z, SaiGautam G, Kolli SK, Chotard J-N, Cheetham AK, Masquelier C, Canepa P (2020) Phase Behavior in Rhombohedral NaSiCON Electrolytes and Electrodes. Chem Mater 32(18):7908–7920. https://doi.org/10.1021/acs.chemmater.0c02695
Roy S, Kumar PP (2013) Influence of Si/P ordering on Na+ transport in NASICONs. Phys Chem Chem Phys 15(14):4965–4969. https://doi.org/10.1039/C3CP43376E
Guin M, Tietz F (2015) Survey of the transport properties of sodium superionic conductor materials for use in sodium batteries. J Power Sources 273:1056–1064. https://doi.org/10.1016/j.jpowsour.2014.09.137
Oh JAS, He L, Plewa A, Morita M, Zhao Y, Sakamoto T, Song X, Zhai W, Zeng K, Lu L (2019) Composite NASICON (Na3Zr2Si2PO12) solid-state electrolyte with enhanced Na+ ionic conductivity: effect of liquid phase sintering. ACS Appl Mater Interfaces 11(43):40125–40133. https://doi.org/10.1021/acsami.9b14986
Boilot JP, Collin G, Colomban P (1987) Crystal structure of the true nasicon: Na3Zr2Si2PO12. Mater Res Bull 22(5):669–676. https://doi.org/10.1016/0025-5408(87)90116-4
Ran L, Baktash A, Li M, Yin Y, Demir B, Lin T, Li M, Rana M, Gentle I, Wang L, Searles DJ, Knibbe R (2021) Sc, Ge co-do** NASICON boosts solid-state sodium ion batteries’ performance. Energy Storage Mater 40:282–291. https://doi.org/10.1016/j.ensm.2021.05.017
Naqash S (2019) Sodium ion conducting ceramics for sodium ion batteries, Forschungszentrum Jülich GmbH, Institut für Energie- und Klimaforschung. Forschungszentrum Jülich GmbH, Zentralbibliothek, Verlag, Germany
Deng Y, Eames C, Nguyen LHB, Pecher O, Griffith KJ, Courty M, Fleutot B, Chotard J-N, Grey CP, Islam MS, Masquelier C (2018) Crystal structures, local atomic environments, and ion diffusion mechanisms of scandium-substituted sodium superionic conductor (NASICON) solid electrolytes. Chem Mater 30(8):2618–2630. https://doi.org/10.1021/acs.chemmater.7b05237
Mazza D (2001) Modeling ionic conductivity in Nasicon structures. J Solid State Chem 156(1):154–160. https://doi.org/10.1006/jssc.2000.8975
Baur WH, Dygas JR, Whitmore DH, Faber J (1986) Neutron powder diffraction study and ionic conductivity of Na2Zr2SiP2O12 and Na3Zr2Si2PO12. Solid State Ionics 18–19:935–943. https://doi.org/10.1016/0167-2738(86)90290-0
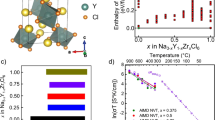
Zhang Z, Zou Z, Kaup K, **ao R, Shi S, Avdeev M, Hu Y-S, Wang D, He B, Li H, Huang X, Nazar LF, Chen L (2019) Correlated migration invokes higher Na+-Ion conductivity in NaSICON-type solid electrolytes. Adv Energy Mater 9(42):1902373. https://doi.org/10.1002/aenm.201902373
Kumar PP, Yashonath S (2006) Ionic conduction in the solid state. J Chem Sci 118(1):135–154. https://doi.org/10.1007/BF02708775
Losilla ER, Aranda MAG, Bruque S, París MA, Sanz J, West AR (1998) Understanding Na mobility in NASICON materials: a rietveld, 23Na and 31P MAS NMR, and impedance study. Chem Mater 10(2):665–673. https://doi.org/10.1021/cm970648j
Huang C, Yang G, Yu W, Xue C, Zhai Y, Tang W, Hu N, Wen Z, Lu L, Song S (2021) Gallium-substituted Nasicon Na3Zr2Si2PO12 solid electrolytes. J Alloys Compd 855(2):157501
Park H, Jung K, Nezafati M, Kim C-S, Kang B, Appl ACS (2016) Sodium ion diffusion in Nasicon (Na3Zr2Si2PO12) solid electrolytes: effects of excess sodium. Mater Interfaces 8(41):27814–27824. https://doi.org/10.1021/acsami.6b09992
Saito Y, Ado K, Asai T, Kageyama H, Nakamura O (1992) Ionic conductivity of NASICON-type conductors Na1. 5M0. 5Zr1. 5(PO4)3 (M: Al3+, Ga3+, Cr3+, Sc3+, Fe3+, In3+, Yb3+, Y3+). Solid State Ionics 58(3–4):327–331. https://doi.org/10.1016/0167-2738(92)90136-D
Roy S, Kumar PP (2013) Influence of Cationic ordering on ion transport in NASICONs: Molecular dynamics study. Solid State Ionics 253:217–222. https://doi.org/10.1016/j.ssi.2013.09.030
Fuentes R, Figueiredo F, Marques F, Franco J (2001) Influence of microstructure on the electrical properties of NASICON materials. Solid State Ionics 140(1–2):173–179. https://doi.org/10.1016/S0167-2738(01)00701-9
Samiee M, Radhakrishnan B, Rice Z, Deng Z, Meng YS, Ong SP, Luo J (2017) Divalent-doped Na3Zr2Si2PO12 natrium superionic conductor: Improving the ionic conductivity via simultaneously optimizing the phase and chemistry of the primary and secondary phases. J Power Sources 347:229–237. https://doi.org/10.1016/j.jpowsour.2017.02.042
Bogusz W, Krok F, Jakubowski W (1981) Bulk and grain boundary electrical conductivities of NASICON. Solid State Ionics 2(3):171–174. https://doi.org/10.1016/0167-2738(81)90175-2
Bohnke O, Ronchetti S, Mazza D (1999) Conductivity measurements on nasicon and nasicon-modified materials. Solid State Ionics 122(1–4):127–136. https://doi.org/10.1016/S0167-2738(99)00062-4
Lee JS, Chang CM, Lee YI, Lee JH, Hong SH (2004) Spark plasma sintering (SPS) of NASICON ceramics. J Am Ceram Soc 87(2):305–307. https://doi.org/10.1111/j.1551-2916.2004.00305
Yoldas BE, Lloyd I (1983) Nasicon formation by chemical polymerization. Mater Res Bull 18(10):1171–1177. https://doi.org/10.1016/0025-5408(83)90019-3
Lu Y, Alonso JA, Yi Q, Lu L, Wang ZL, Sun C (2019) A high‐performance monolithic solid‐state sodium battery with Ca2+ doped Na3Zr2Si2PO12 electrolyte. Adv Energy Mater 9(28):1901205. https://doi.org/10.1002/aenm.201901205
Nishio A, Shirai N, Minami H, Izumi H, Inoishi A, Okada S (2021) Effect of Na3BO3 Addition into Na3V2(PO4)3 Single-Phase All-Solid-State Batteries. Electrochemistry 21:244–249. https://doi.org/10.5796/electrochemistry.21-00023
Noi K, Suzuki K, Tanibata N, Hayashi A, Tatsumisago M (2018) Liquid‐phase sintering of highly Na+ ion conducting Na3Zr2Si2PO12 ceramics using Na3BO3 additive. J Am Ceram Soc 101(3):1255–1265. https://doi.org/10.1111/jace.15288
Suzuki K, Noi K, Hayashi A, Tatsumisago M (2018) Low temperature sintering of Na1+xZr2SixP3−xO12 by the addition of Na3BO3. Scr Mater 145:67–70. https://doi.org/10.1016/j.scriptamat.2017.10.010
Leng H, Nie J, Luo J (2019) Combining cold sintering and Bi2O3-Activated liquid-phase sintering to fabricate high-conductivity Mg-doped NASICON at reduced temperatures. J Materiomics 5(2):237–246. https://doi.org/10.1016/j.jmat.2019.02.005
Cao XG, Zhang XH, Tao T, Zhang HY (2020) Effects of antimony tin oxide (ATO) additive on the properties of Na3Zr2Si2PO12 ceramic electrolytes. Ceram Int 46(6):8405–8412. https://doi.org/10.1016/j.ceramint.2019.12.074
Chen D, Luo F, Gao L, Zhou W, Zhu D (2017) Influence of Indium-Tin Oxide Additive on the Sintering Process and Conductivity of Na3Zr2Si2PO12 Solid Electrolyte. J Electron Mater 46(11):6367–6372. https://doi.org/10.1007/s11664-017-5674-7
Wang X, Liu Z, Tang Y, Chen J, Wang D, Mao Z (2021) Low temperature and rapid microwave sintering of Na3Zr2Si2PO12 solid electrolytes for Na-Ion batteries. J Power Sources 481:228924. https://doi.org/10.1016/j.jpowsour.2020.228924
Thirupathi R, Omar S (2021) J Phys Chem C 125(50):27723
Narayanan S, Reid S, Butler S, Thangadurai V (2019) Sintering temperature, excess sodium, and phosphorous dependencies on morphology and ionic conductivity of NASICON Na3Zr2Si2PO12. Solid State Ionics 331:22–29. https://doi.org/10.1016/j.ssi.2018.12.003
Essoumhi A, Favotto C, Mansori M, Satre P (2004) Synthesis and characterization of a NASICON series with general formula Na2. 8Zr2− ySi1. 8− 4yP1. 2+ 4yO12 (0⩽ y⩽ 0.45). J Solid State Chem 177(12):4475–4481. https://doi.org/10.1016/j.jssc.2004.09.026
Martucci A, Sartori S, Guglielmi M, Di Vona ML, Licoccia S, Traversa E (2002) NMR and XRD study of the influence of the P precursor in sol-gel synthesis of NASICON powders and films. J Eur Ceram Soc 22(12):1995–2000. https://doi.org/10.1016/S0955-2219(01)00536-2
Clearfield A, Subramanian MA, Wang W, Jerus P (1983) The use of hydrothermal procedures to synthesize NASICON and some comments on the stoichiometry of NASICON phases. Solid State Ionics 9–10(2):895–902. https://doi.org/10.1016/0167-2738(83)90108-X
Clearfield A, Jerus P, Cotman RN (1981) Hydrothermal and solid-state synthesis of sodium zirconium silicophosphates. Solid State Ionics 5:301–304. https://doi.org/10.1016/0167-2738(81)90252-6
Ignaszak A, Pasierb P, Gajerski R, Komornicki S (2005) Synthesis and properties of Nasicon-type materials. Thermochim Acta 426(1):7–14. https://doi.org/10.1016/j.tca.2004.07.002
Fuentes R, Figueiredo F, Soares M, Marques F (2005) Submicrometric NASICON ceramics with improved electrical conductivity obtained from mechanically activated precursors. J Eur Ceram Soc 25(4):455–462. https://doi.org/10.1016/j.jeurceramsoc.2004.02.019
Dhas NA, Patil KC (1994) Controlled combustion synthesis and properties of fine-particle NASICON materials. J Mater Chem 4(3):491–497. https://doi.org/10.1039/JM9940400491
McEntire BJ, Bartlett R, Miller G, Gordon R (1983) Effect of decomposition on the densification and properties of nasicon ceramic electrolytes. J Am Ceram Soc 66(10):738–742. https://doi.org/10.1111/j.1151-2916.1983.tb10541.x
Bell NS, Edney C, Wheeler JS, Ingersoll D, Spoerke ED (2014) The Influences of Excess Sodium on Low‐Temperature NaSICON Synthesis. J Am Ceram Soc 97(12):3744–3748. https://doi.org/10.1111/jace.13167
Gasmi N, Gharbi N, Zarrouk H, Barboux P, Morineau R, Livage J (1995) Comparison of different synthesis methods for Nasicon ceramics. J Sol-Gel Sci Technol 4(3):231–237. https://doi.org/10.1007/BF00488378
Ahmad A, Wheat T, Kuriakose A, Canaday J, McDonald A (1987) Dependence of the properties of Nasicons on their composition and processing. Solid State Ionics 24(1):89–97. https://doi.org/10.1016/0167-2738(87)90070-1
Warhus U, Maier J, Rabenau A (1988) Thermodynamics of NASICON (Na1+xZr2SixP3−xO12). J Solid State Chem 72(1):113–125. https://doi.org/10.1016/0022-4596(88)90014-X
Zhang S, Quan B, Zhao Z, Zhao B, He Y, Chen W (2004) Preparation and characterization of NASICON with a new sol–gel process. Mater Lett 58(1):226–229. https://doi.org/10.1016/S0167-577X(03)00450-6
Yadav P, Bhatnagar M (2012) Structural studies of NASICON material of different compositions by sol–gel method. Ceram Int 38(2):1731–1735. https://doi.org/10.1016/j.ceramint.2011.09.022
Traversa E, Aono H, Sadaoka Y, Montanaro L (2000) Electrical properties of sol–gel processed NASICON having new compositions. Sens Actuators B 65(1–3):204–208. https://doi.org/10.1016/S0925-4005(99)00293-2
Naqash S, Ma Q, Tietz F, Guillon O (2017) Na3Zr2(SiO4)2(PO4) prepared by a solution-assisted solid-state reaction. Solid State Ionics 302:83–91. https://doi.org/10.1016/j.ssi.2016.11.004
Okubo K, Wang H, Hayashi K, Inada M, Enomoto N, Hasegawa G, Osawa T, Takamura H (2018) A dense NASICON sheet prepared by tape-casting and low temperature sintering. Electrochim Acta 278:176–181. https://doi.org/10.1016/j.electacta.2018.05.020
Grady ZM, Tsuji K, Ndayishimiye A, Hwan-Seo J, Randall CA (2020) Densification of a Solid-State NASICON Sodium-Ion Electrolyte Below 400° C by Cold Sintering with a Fused Hydroxide Solvent. Appl ACS Energy Mater 3(5):4356–4366. https://doi.org/10.1021/acsaem.0c00047
Grasso S, Biesuz M, Zoli L, Taveri G, Duff AI, Ke D, Jiang A, Reece MJ (2020) A review of cold sintering processes. Adv Appl Ceram 119(3):115–143. https://doi.org/10.1080/17436753.2019.1706825
da Silva JGP, Bram M, Laptev AM, Gonzalez-Julian J, Ma Q, Tietz F, Guillon O (2019) Sintering of a sodium based NASICON electrolyte: a comparative study between cold, field assisted and conventional sintering methods. J Eur Ceram Soc 39(8):2697–2702. https://doi.org/10.1016/j.jeurceramsoc.2019.03.023
Kuriakose A, Wheat T, Ahmad A, Dirocco J (1984) Synthesis, sintering, and microstructure of NASICONS. J Am Ceram Soc 67(3):179–183. https://doi.org/10.1111/j.1151-2916.1984.tb19737.x
Kwon OJ, Yoon DN (1980) The liquid phase sintering of W-Ni. Sintering processes, Materials Science Research. Plenum Press, New York
Lee S-M, Lee S-T, Lee D-H, Lee S-H, Han S-S, Lim S-K (2015) Effect of particle size on the density and ionic conductivity of Na3Zr2Si2PO12 NASICON. J Ceram Process Res 16(1):49–53. https://doi.org/10.36410/jcpr.2015.16.1.49
Choi S-D, Park J-W (1996) Preparation of NASICON Powder and Electrolyte. Sens Mater 8(8):505–511
Ceramatec, Inc. (1980) J Power Sources 5 (4):413
Fuentes R, Figueiredo F, Marques F, Franco J (2001) Processing and electrical properties of NASICON prepared from yttria-doped zirconia precursors. J Eur Ceram Soc 21(6):737–743. https://doi.org/10.1016/S0955-2219(00)00264-8
Von Alpen U, Bell MF, Höfer HH (1981) Compositional dependence of the electrochemical and structural parameters in the Nasicon system (Na1+xSixZr2P3−xO12). Solid State Ionics 3–4:215–218. https://doi.org/10.1016/0167-2738(81)90085-0
Yde-Andersen S, Lundsgaard JS, Møller L, Engell J (1984) Properties of NASICON electrolytes prepared from alkoxide derived gels: ionic conductivity, durability in molten sodium and strength test data. Solid State Ionics 14(1):73–79. https://doi.org/10.1016/0167-2738(84)90014-6
Zhang Q, Liang F, Qu T, Yao Y, Ma W, Yang B, Dai Y (2018) Effect on ionic conductivity of Na3+ xZr2-xMxSi2PO12 (M= Y, La) by do** rare-earth elements (2018) IOP Conference Series: Materials Science and Engineering, IOP Publishing, 423:012122
Khakpour Z (2016) Influence of M: Ce4+, Gd3+ and Yb3+ substituted Na3+xZr2-xMxSi2PO12 solid NASICON electrolytes on sintering, microstructure and conductivity. Electrochim Acta 196:337–347. https://doi.org/10.1016/2Fj.electacta.2016.02.199
Shao Y, Zhong G, Lu Y, Liu L, Zhao C, Zhang Q, Hu Y-S, Yang Y, Chen L (2019) A novel NASICON-based glass-ceramic composite electrolyte with enhanced Na-ion conductivity. Energy Storage Mater 23:514–521. https://doi.org/10.1016/j.ensm.2019.04.009
Goodenough JB, Hong H-P, Kafalas J (1976) Fast Na+-ion transport in skeleton structures. Mater Res Bull 11(2):203–220. https://doi.org/10.1016/0025-5408(76)90077-5
Banik A, Famprikis T, Ghidiu M, Ohno S, Kraft MA, Zeier WG (2021) On the underestimated influence of synthetic conditions in solid ionic conductors. Chem Sci 12(18):6238–6263. https://doi.org/10.1039/D0SC06553F
Yao Z, Zhu K, Zhu K, Zhang J, Li X, Chen J, Wang J, Yan K, Liu J (2021) Co-Precipitation Synthesis and Electrochemical Properties of NASICON-Type Li1. 3Al0.3Ti1.7(PO4)3 Solid Electrolytes. J Mater Sci: Mater Electron 32:24834–24844. https://doi.org/10.1007/s10854-021-06943-x
Dai H, Xu W, Hu Z, Chen Y, Wei X, Yang B, Chen Z, Gu J, Yang D, **e F (2020) Effective approaches of improving the performance of chalcogenide solid electrolytes for all-solid-state sodium-ion batteries. Front Energy Res 8:97. https://doi.org/10.3389/fenrg.2020.00097
**e B, Jiang D, Wu J, Feng T, **a J, Nian H (2016) Effect of substituting Ce for Zr on the electrical properties of NASICON materials. J Phys Chem Solids 88:104–108. https://doi.org/10.1016/j.jpcs.2015.10.003
Yang J, Wan H-L, Zhang Z-H, Liu G-Z, Xu X-X, Hu Y-S, Yao X-Y (2018) NASICON-structured Na3.1Zr1.95Mg0.05Si2PO12 solid electrolyte for solid-state sodium batteries. Rare Met 37(6):480–487. https://doi.org/10.1007/s12598-018-1020-3
Song S, Duong HM, Korsunsky AM, Hu N, Lu L (2016) A Na+ superionic conductor for room-temperature sodium batteries. Sci Rep 6(1):1–10. https://doi.org/10.1038/srep32330
Shen L, Yang J, Liu G, Avdeev M, Yao X (2021) High ionic conductivity and dendrite-resistant NASICON solid electrolyte for all-solid-state sodium batteries. Mater Today Energy 20:100691
Santhoshkumar B, Rao PL, Ramanathan K, Bera A, Yusuf S, Hathwar VR, Pahari B (2021) Structure and ionic conductivity of Na3+xSc2SixP3-xO12 (x= 0.0, 0.2, 0.4, 0.8) NASICON materials: A combined neutron diffraction, MAS NMR and impedance study. Solid State Sci 111:106470
Chen D, Luo F, Zhou W, Zhu D (2018) Influence of Nb5+, Ti4+, Y3+ and Zn2+ doped Na3Zr2Si2PO12 solid electrolyte on its conductivity. J Alloys Compd 757:348–355. https://doi.org/10.1016/j.jallcom.2018.05.116
Yang J, Liu G, Avdeev M, Wan H, Han F, Shen L, Zou Z, Shi S, Hu Y-S, Wang C (2020) Ultrastable all-solid-state sodium rechargeable batteries. ACS Energy Lett 5(9):2835–2841. https://doi.org/10.1021/acsenergylett.0c01432
Sun F, **ang Y, Sun Q, Zhong G, Banis MN, Li W, Liu Y, Luo J, Li R, Fu R (2021) Insight into Ion Diffusion Dynamics/Mechanisms and Electronic Structure of Highly Conductive Sodium-Rich Na3+xLaxZr2–xSi2PO12 (0≤ x≤ 0.5) Solid-State Electrolytes. ACS Appl Mater Interfaces 13(11):13132–13138. https://doi.org/10.1021/acsami.0c21882
Ruan Y, Song S, Liu J, Liu P, Cheng B, Song X, Battaglia V (2017) Improved structural stability and ionic conductivity of Na3Zr2Si2PO12 solid electrolyte by rare earth metal substitutions. Ceram Int 43(10):7810–7815. https://doi.org/10.1016/j.ceramint.2017.03.095
Hayashi K, Shima K, Sugiyama F (2013) A mixed aqueous/aprotic sodium/air cell using a NASICON ceramic separator. J Electrochem Soc 160(9):A1467–A1472. https://doi.org/10.1149/2.067309jes
Liu Y, Liu L, Peng J, Zhou X, Liang D, Zhao L, Su J, Zhang B, Li S, Zhang N, Ma Q, Tietz F (2022) A niobium-substituted sodium superionic conductor with conductivity higher than 5.5 mS cm−1 prepared by solution-assisted solid-state reaction method. J Power Sources 518:230765. https://doi.org/10.1016/j.jpowsour.2021.230765
Ma Q, Tsai C-L, Wei X-K, Heggen M, Tietz F, Irvine JT (2019) Room temperature demonstration of a sodium superionic conductor with grain conductivity in excess of 0.01 S cm−1 and its primary applications in symmetric battery cells. J Mater Chem A 7(13):7766–7776. https://doi.org/10.1039/C9TA00048H
Zhou M, Ahmad A (2007) Synthesis, processing and characterization of nasicon solid electrolytes for CO2 sensing applications. Sens Actuators B 122(2):419–426. https://doi.org/10.1016/j.snb.2006.06.011
Lalère F, Leriche J-B, Courty M, Boulineau S, Viallet V, Masquelier C, Seznec V (2014) An all-solid state NASICON sodium battery operating at 200 °C. J Power Sources 247:975–980. https://doi.org/10.1016/j.jpowsour.2013.09.051
Porkodi P, Yegnaraman V, Kamaraj P, Kalyanavalli V, Jeyakumar D (2008) Synthesis of NASICON- A molecular precursor-based approach. Chem Mater 20(20):6410–6419. https://doi.org/10.1021/cm800208k
Ortiz-Mosquera JF, Nieto-Munoz AM, Rodrigues AC (2020) New Na1+ xGe2(SiO4)x(PO4)3–x NASICON Series with Improved Grain and Grain Boundary Conductivities. ACS Appl Mater Interfaces 12(12):13914–13922. https://doi.org/10.1021/acsami.9b23065
Park H, Kang M, Park Y-C, Jung K, Kang B (2018) Improving ionic conductivity of NASICON (Na3Zr2Si2PO12) at intermediate temperatures by modifying phase transition behavior. J Power Sources 399:329–336. https://doi.org/10.1016/j.jpowsour.2018.07.113
Pal SK, Saha R, Kumar GV, Omar S (2020) Designing High Ionic Conducting NASICON-type Na3Zr2Si2PO12 Solid-Electrolytes for Na-Ion Batteries. J Phys Chem C 124(17):9161–9169. https://doi.org/10.1021/acs.jpcc.0c00543
Srikanth V, Subbarao EC, Agrawal DK, Huang CY, Roy R, Rao GV (1991) Thermal expansion anisotropy and acoustic emission of NaZr2P3O12 family ceramics. J Am Ceram Soc 74(2):365–368. https://doi.org/10.1111/j.1151-2916.1991.tb06888.x
Naqash S, Gerhards M-T, Tietz F, Guillon O (2018) Coefficients of Thermal Expansion of Al-and Y-Substituted NaSICON Solid Solution Na3+2xAlxYxZr2−2xSi2PO12. Batteries 4(3):33. https://doi.org/10.3390/batteries4030033
Oota T, Yamai I (1986) Thermal Expansion Behavior of NaZr2(PO4)3 Type Compounds. J Am Ceram Soc 69(1):1–6. https://doi.org/10.1111/j.1151-2916.1986.tb04682.x
Kaus M, Guin M, Yavuz M, Knapp M, Tietz F, Guillon O, Ehrenberg H, Indris S (2017) Fast Na+ ion conduction in NASICON-type Na3.4Sc2(SiO4)0.4(PO4)2.6 observed by 23Na NMR relaxometry. J Phys Chem C 121(3):1449–1454. https://doi.org/10.1021/acs.jpcc.6b10523
Alamo J, Roy R (1984) Ultralow-expansion ceramics in the system Na2O-ZrO2-P2O5-SiO2. J Am Ceram Soc 67(5):c78–c80
Pet’kov V, Asabina E, Shchelokov I (2013) Thermal expansion of NASICON materials. Inorg Mater 49(5):502–506. https://doi.org/10.1134/S0020168513050117
Guin M, Tietz F, Guillon O (2016) New promising NASICON material as solid electrolyte for sodium-ion batteries: Correlation between composition, crystal structure and ionic conductivity of Na3+ xSc2SixP3− xO12. Solid State Ionics 293:18–26. https://doi.org/10.1016/j.ssi.2016.06.005
Cleveland J, Bradt R (1978) Grain size/microcracking relations for pseudobrookite oxides. J Am Ceram Soc 61(11–12):478–481. https://doi.org/10.1111/j.1151-2916.1978.tb16121.x
Jackman SD, Cutler RA (2012) Effect of microcracking on ionic conductivity in LATP. J Power Sources 218:65–72. https://doi.org/10.1016/j.jpowsour.2012.06.081
Bradt RC (1995) Low expansion materials. Ceram Trans 52:5–18
Min K (2021) High-throughput Ab initio investigation of the elastic properties of inorganic electrolytes for all-solid-state Na-ion batteries. J Electrochem Soc 168(3):030541. https://doi.org/10.1149/1945-7111/abf015
Monroe C, Newman J (2005) The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J Electrochem Soc 152(2):A396–A404. https://doi.org/10.1149/1.1850854
Tang B, Jaschin PW, Li X, Bo S-H, Zhou Z (2020) Mater Today 41:200
Nonemacher JF, Naqash S, Tietz F, Malzbender J (2019) Micromechanical assessment of Al/Y-substituted NASICON solid electrolytes. Ceram Int 45(17):21308–21314. https://doi.org/10.1016/j.ceramint.2019.07.114
Kalnaus S, Amin R, Parish C, Parejiya A, Essehli R, Westover A, Tsai W-Y, Nanda J, Belharouak I (2021) Appl ACS Energy Mater 4(10):11684
Valle JM, Huang C, Tatke D, Wolfenstine J, Go W, Kim Y, Sakamoto J (2021) Characterization of hot-pressed von Alpen type NASICON ceramic electrolytes. Solid State Ionics 369:115712
Kim J, Jo SH, Bhavaraju S, Eccleston A, Kang SO (2015) Low temperature performance of sodium–nickel chloride batteries with NaSICON solid electrolyte. J Electroanal Chem 759:201–206. https://doi.org/10.1016/j.jelechem.2015.11.022
Ruan Y, Guo F, Liu J, Song S, Jiang N, Cheng B (2019) Optimization of Na3Zr2Si2PO12 ceramic electrolyte and interface for high performance solid-state sodium battery. Ceram Int 45(2):1770–1776. https://doi.org/10.1016/j.ceramint.2018.10.062
Uchida Y, Hasegawa G, Shima K, Inada M, Enomoto N, Akamatsu H, Hayashi K (2019) Insights into sodium ion transfer at the Na/NASICON interface improved by uniaxial compression. ACS Appl Energy Mater 2(4):2913–2920. https://doi.org/10.1021/acsaem.9b00250
Zhu Y, He X, Mo Y (2015) Origin of Outstanding Stability in the Lithium Solid Electrolyte Materials: Insights from Thermodynamic Analyses Based on First-Principles Calculations. ACS Appl Mater Interfaces 7(42):23685–23693. https://doi.org/10.1021/acsami.5b07517
Zhu Y, He X, Mo Y (2016) First principles study on electrochemical and chemical stability of solid electrolyte–electrode interfaces in all-solid-state Li-ion batteries. J Mater Chem A 4(9):3253–3266. https://doi.org/10.1039/C5TA08574H
Lacivita V, Wang Y, Bo S-H, Ceder G (2019) Ab initio investigation of the stability of electrolyte/electrode interfaces in all-solid-state Na batteries. J Mater Chem A 7(14):8144–8155. https://doi.org/10.1039/C8TA10498K
Fu H, Yin Q, Huang Y, Sun H, Chen Y, Zhang R, Yu Q, Gu L, Duan J, Luo W (2019) Reducing interfacial resistance by Na-SiO2 composite anode for NASICON-based solid-state sodium battery. ACS Mater Lett 2(2):127–132. https://doi.org/10.1021/acsmaterialslett.9b00442
Shi C, Wang L, Chen X, Li J, Wang S, Wang J, ** H (2022) Challenges of layer-structured cathodes for sodium-ion batteries. Nanoscale Horiz 7(4):338–351. https://doi.org/10.1039/D1NH00585E
Wei F, Zhang Q, Zhang P, Tian W, Dai K, Zhang L, Mao J, Shao G (2021) Review—research progress on layered transition metal oxide cathode materials for sodium ion batteries. J Electrochem Soc 168(5):050524. https://doi.org/10.1149/1945-7111/abf9bf
Bhange DS, Anang DA, Ali G, Park J-H, Kim J-Y, Bae J-H, Yoon WY, Chung KY, Nam K-W (2020) NaFeSnO4: Tunnel structured anode material for rechargeable sodium-ion batteries. Electrochem Commun 121:106873. https://doi.org/10.1016/j.elecom.2020.106873
Nuti M, Spada D, Quinzeni I, Capelli S, Albini B, Galinetto P, Bini M (2020) From tunnel NMO to layered polymorphs oxides for sodium ion batteries. SN Appl Sci 2(11):1893. https://doi.org/10.1007/s42452-020-03607-z
Barpanda P, Lander L, Nishimura S-I, Yamada A (2018) Polyanionic Insertion Materials for Sodium-Ion Batteries. Adv Energy Mater 8(17):1703055. https://doi.org/10.1002/aenm.201703055
Niu Y, Zhang Y, Xu M (2019) A review on pyrophosphate framework cathode materials for sodium-ion batteries. J Mater Chem A 7(25):15006–15025. https://doi.org/10.1039/C9TA04274A
Wang D, Bie X, Fu Q, Dixon D, Bramnik N, Hu YS, Fauth F, Wei Y, Ehrenberg H, Chen G, Du F (2017) Sodium vanadium titanium phosphate electrode for symmetric sodium-ion batteries with high power and long lifespan. Nat Commun 8:15888. https://doi.org/10.1038/ncomms15888
Lan T, Tsai C-L, Tietz F, Wei X-K, Heggen M, Dunin-Borkowski RE, Wang R, **ao Y, Ma Q, Guillon O (2019) Room-temperature all-solid-state sodium batteries with robust ceramic interface between rigid electrolyte and electrode materials. Nano Energy 65:104040. https://doi.org/10.1016/j.nanoen.2019.104040
Liu L, Qi X, Ma Q, Rong X, Hu Y-S, Zhou Z, Li H, Huang X, Chen L (2016) Toothpaste-like electrode: A novel approach to optimize the interface for solid-state sodium-ion batteries with ultralong cycle life. ACS Appl Mater Interfaces 8(48):32631–32636. https://doi.org/10.1021/acsami.6b11773
Zhang Z, Xu K, Rong X, Hu Y-S, Li H, Huang X, Chen L (2017) Na3.4Zr1.8Mg0.2Si2PO12 filled poly (ethylene oxide)/Na(CF3SO2)2N as flexible composite polymer electrolyte for solid-state sodium batteries. J Power Sources 372:270–275. https://doi.org/10.1016/j.jpowsour.2017.10.083
Płcharski J, Weiczorek W (1988) PEO based composite solid electrolyte containing nasicon. Solid State Ionics 28:979–982. https://doi.org/10.1016/0167-2738(88)90315-3
Wang X, Chen J, Mao Z, Wang D (2022) Effective resistance to dendrite growth of NASICON solid electrolyte with lower electronic conductivity. Chem Eng J 427:130899. https://doi.org/10.1016/j.cej.2021.130899
Goodenough JB, Singh P (2015) Solid electrolytes in rechargeable electrochemical cells. J Electrochem Soc 162(14):A2387–A2392. https://doi.org/10.1149/2.0021514jes
Acknowledgements
The authors gratefully acknowledge the support from the Department of Science and Technology, Government of India (grant no. EMR/2016/005438) toward the funding of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, K., Chakraborty, A., Thirupathi, R. et al. Recent advances in NASICON-type oxide electrolytes for solid-state sodium-ion rechargeable batteries. Ionics 28, 5289–5319 (2022). https://doi.org/10.1007/s11581-022-04765-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-022-04765-3