Abstract
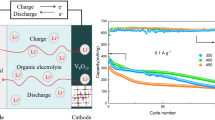
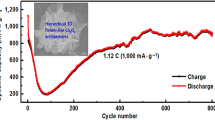
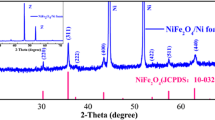
3D flower-like Li0.36V6O13 has been fabricated via a facile solvothermal method using C2H5OH, V2O5, and LiNO3 as raw materials. The microstructure of the sample was characterized by XRD, FESEM, TEM, and XPS. The lithium storage performance of the sample was investigated by CV, EIS, and charge/discharge test. The results demonstrated that the Li0.36V6O13 sample exhibited greatly improved electrochemical performance as compared with the pristine V6O13. For example, when cycled at 0.1 C for 50 cycles, the capacity retention of the Li0.36V6O13 is 97% much higher than that (57%) of the pristine V6O13. The improvement of the cycle performance of Li0.36V6O13 is attributed to its superior structural reversibility, fewer number of phase transitions during the discharge/charge process, improved electrical conductivity, and enhanced Li+ diffusivity.






Similar content being viewed by others
References
Yoo EJ, Kim J, Hosono E, Zhou HS, Kudo T, Honma I (2008) Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett 8:2277–2282
Hu LH, Kumar P (2015) Sulphur-reduced self-assembly of flower-like vanadium pentoxide as superior cathode material for Li-ion battery. J Alloys Compd 655:79–85
Kumar P, Wu FY, Chou T, Hu LH (2015) Chemically modified morphologies of vanadium pentoxide as superior cathode material for lithium ion battery. J Alloys Compd 632:126–132
Wang HL, Yang Y, Liang YY, Robinson JT, Li YG, Jackson A, Cui Y, Dai HJ (2011) Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur-battery cathode material with high capacity and cycling stability. Nano Lett 11:2644–2647
Liu J, **a H, Xue DF, Li L (2009) Double-shelled nanocapsules of V2O5 based composites as high-performance anode and cathode materials for Li ion batteries. J Am Chem Soc 131:12086–12087
Menetrier M, Levasseur A, Delmas C (1989) Utilization of V6O13 as the positive electrode in lithium batteries. Mater Sci Eng B 3:103–107
Schmitt T, Augustsson A, Nordgren J, Duda LC, Höwing J, Gustafsson T, Schwingenschlögl U, Eyert V (2005) Electronic structure of Li-inserted V6O13 battery cathodes: Rigid band behavior and effects of hybridization, Appl Phys Lett 86 :064101-1-064101-3
Li H, He P, Wang Y, Hosono E, Zhou H (2011) High-surface vanadium oxides with large capacities for lithium-ion batteries: from hydrated aerogel to nanocrystalline VO2(B), V6O13 and V2O5. J Mater Chem 21:10999–11009
Björk H, Lidin S, Gustafsson T, Thomas JO (2010) Superlattice formation on lithiated vanadium oxide phases Li0.67V6O13 and Li1V6O13. Acta Crystallogr 57:759–765
Höwing J, Gustafsson T, Thomas JO (2010) Low-temperature structure of V6O13. Acta Crystallogr 59:747–752
Xu SX, Cen DC, Gao PB, Huang T, Bao ZH (2018) 3D interconnected V6O13 nanosheets grown on carbonized textile via a seed-assisted hydrothermal process as high-performance flexible cathodes for lithium-ion Batteries. Nanoscale Res Lett 13:1–7
Zheng SS, Li XR, Yan BY, Hu Q, Xu YX, **ao X, Xue HG, Pang H (2017) Transition-metal (Fe, Co, Ni) based metal-organic frameworks for electrochemical energy storage. Adv Energy Mater 7:1602733
Geng PB, Zheng SS, Tang H, Zhu RM, Zhang L, Cao S, Xue HG, Pang H (2018) Transition metal sulfides based on graphene for electrochemical energy storage. Adv Energy Mater 8:1703259
Mancini M, Axmann P, Gabrielli G, Kinyanjui M, Kaiser U, Wohlfhrt-Mehrens M (2016) A high-voltage and high-capacity Li1+xNi0.5Mn1.5O4 cathode material: from synthesis to full lithium-ion cells. Chemsuschem 9:1843–1849
Gabrielli G, Marinaro M, Mancini M, Axmann P, Wohlfhrt-Mehrens M (2017) A new approach for compensating the irreversible capacity loss of high-energy Si/C|LiNi0.5Mn1.5O4 lithium-ion batteries. J Power Sources 351:35–44
Zhan SY, Wang CZ, Nikolowski K, Ehrenberg H, Chen G, Wei YJ (2009) Electrochemical properties of Cr doped V2O5, between 3.8 V and 2.0 V. Solid State Ionics 180:1198–1203
West K, Zachau-Christiansen B, Jacobsen T, Atlung S (1985) V6O13, As cathode material for lithium cells. J Power Sources 14:235–245
Wertheim GK, Attekum PT, Basu S (1980) Electronic structure of lithium graphite. Solid State Commun 33:1127–1130
Ichimura K, Sano M (1991) Electrical conductivity of layered transition-metal phosphorus trisulfide crystals. Synth Met 45:203–211
Mezentzeff P, Lifshitz Y, Rabalais JW (1990) Compositional and chemical modifications of V2O5 and NaVO3 induced by N2+ bombardment. Nucl Instrum Methods Phys Res Sect B 44:296–301
Lv TT, Zou ZG, Li YW, Li SY, Zhang YJ (2018) Hydrothermal synthesis of high specific capacity Al/Na co-doped V6O13 cathode material for lithium-ion battery. J Electroanal Chem 829:42–50
Simões M, Surace Y, Yoon S, Battaglia C, Pokrant S, Weidenkaff A (2015) Hydrothermal vanadium manganese oxides: anode and cathode materials for lithium-ion batteries. J Power Sources 291:66–74
Mizuno Y, Hosono E, Saito T, Okubo M, Nishio-Hamane D, Oh-ishi K, Kudo T, Zhou HS (2012) Electrospinning synthesis of wire-structured LiCoO/r2/r for electrode materials of high-power Li-ion batteries. J Phys Chem C 116:10774–10780
Luo S, Wang K, Wang JP, Jiang K, Li QQ, Fan SS (2012) Binder-free LiCoO2/carbon nanotube cathodes for high-performance lithium ion batteries. Adv Mater 24:2294–2298
Chernova NA, Roppolo M, Dillon AC, Whittingham MS (2009) Layered vanadium and molybdenum oxides: batteries and electrochromics. J Mater Chem 19:2526–2552
Ding Z, Zhao L, Suo L, Jiao Y, Meng S, Hu YS, Wang Z, Chen L (2011) Towards understanding the effects of carbon and nitrogen-doped carbon coating on the electrochemical performance of Li4Ti5O12 in lithium ion batteries: a combined experimental and theoretical study. Chem Phys 13:15127–15133
Nethravathi C, Rajamathi CR, Rajamathi M, Gautam UK, Wang X, Golberg D (2013) N-doped graphene-VO2 (B) nanosheet-built 3D flower hybrid for lithium ion battery. ACS Appl Mater Interfaces 5:2708–2714
Chakrabarty DK, Guha D, Biswas AB (1976) Electrical properties of vanadium pentoxide doped with lithium and sodium in the α-phase range. J Mater Sci 11:1347–1353
Funding
The authors received financial support from the National Nature Science Foundation of China (project no. 51562006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lv, Tt., Zou, Zg., Li, Yw. et al. Flower-like Li0.36V6O13 with superior cycling stability as a cathode material for lithium-ion batteries. Ionics 26, 1181–1187 (2020). https://doi.org/10.1007/s11581-019-03272-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03272-2