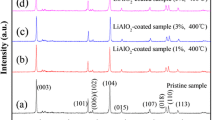
Abstract
The Li[Li0.2Mn0.54Ni0.13Co0.13]O2 coated with CeO2 has been fabricated by an ionic interfusion method. Both the bare and the CeO2-coated samples have a typical layered structure with R-3m and C2/m space group. The results of XRD and TEM images display that the CeO2 coating layer on the precursor could enhance the growth of electrochemically active surface planes ((010), (110), and (100) planes) in the following ionic interfusion process. The results of galvanostatic cycling tests demonstrate that the CeO2-coated sample has a discharge capacity of 261.81 mAh g−1 with an increased initial Coulombic efficiency from 62.4 to 69.1% at 0.05 °C compared with that of bare sample and delivers an improved capacity retention from 71.7 to 83.4% after 100 cycles at 1 °C (1 °C = 250 mA g−1). The results of electrochemical performances confirm that the surface modification sample exhibits less capacity fading, lower voltage decay, and less polarization.








Similar content being viewed by others
References
Yuan B, Wu XF, Chen YX (2013) Adsorption of CO2, CH4, and N2 on ordered mesoporous carbon: approach for greenhouse gases capture and biogas upgrading. Environ Sci Tech Let 47(10):5474–5480. https://doi.org/10.1021/es4000643
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603. https://doi.org/10.1021/cm901452z
Hy S, Liu H, Zhang M, Qian D, Hwang B-J, Meng YS (2016) Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries. Energy Environ Sci 9(6):1931–1954. https://doi.org/10.1039/C5EE03573B
Croy JR, Balasubramanian M, Gallagher KG, Burrell AK (2015) Review of the U.S. department of energy’s “deep dive” effort to understand voltage fade in Li- and Mn-rich cathodes. Accounts Chem Res 48(11):2813–2821. https://doi.org/10.1021/acs.accounts.5b00277
Chen Y, Li X, Zhou X, Yao H, Huang H, Mai YW, Zhou L (2014) Hollow-tunneled graphitic carbon nanofibers through Ni-diffusion-induced graphitization as high-performance anode materials. Energy Environ Sci 7(8):2689–2696. https://doi.org/10.1039/C4EE00148F
Thackeray MM, Wolverton C, Isaacs ED (2012) Electrical energy storage for transportation-approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ Sci 5(7):7854–7863. https://doi.org/10.1039/c2ee21892e
Wang J, Li X, Wang Z, Huang B, Wang Z, Guo H (2014) Nanosized LiVPO4F/graphene composite: a promising anode material for lithium ion batteries. J Power Sources 251:325–330. https://doi.org/10.1016/j.jpowsour.2013.11.095
Yang Z, Guo H, Li X, Wang Z, Yan Z, Wang Y (2016) Natural sisal fibers derived hierarchical porous activated carbon as capacitive material in lithium ion capacitor. J Power Sources 329:339–346. https://doi.org/10.1016/j.jpowsour.2016.08.088
Zheng JC, Han YD, Zhang B, Shen C, Ming L, Zhang JF (2014) Comparative investigation of phosphate-based composite cathode materials for lithium-ion batteries. Acs Appl Mater Inter 6(16):13520–13526. https://doi.org/10.1021/am502601r
He Z, Wang Z, Chen H, Huang Z, Li X, Guo H, Wang R (2015) Electrochemical performance of zirconium doped lithium rich layered Li1.2Mn0.54Ni0.13Co0.13O2 oxide with porous hollow structure. J Power Sources 299:334–341. https://doi.org/10.1016/j.jpowsour.2015.09.025
Buchholz D, Li J, Passerini S, Aquilanti G, Wang D, Giorgetti M (2015) X-ray absorption spectroscopy investigation of lithium-rich, cobalt-poor layered-oxide cathode material with high capacity. Chemelectrochem 2:85–97. https://doi.org/10.1002/celc.201402324
Xu Y, Li X, Wang Z, Guo H, Peng W, Pan W (2016) The enhanced high cut-off voltage electrochemical performances of LiNi0.5Co0.2Mn0.3O2 by the CeO2 modification. Electrochim Acta 219:49–60. https://doi.org/10.1016/j.electacta.2016.09.139
Wang D, Li X, Wang Z, Guo H, Xu Y, Fan Y, Ru J (2016) Role of zirconium dopant on the structure and high voltage electrochemical performances of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries. Electrochim Acta 188:48–56. https://doi.org/10.1016/j.electacta.2015.11.093
Li T, Li X, Wang Z, Guo H (2017) A short process for the efficient utilization of transition-metal chlorides in lithium-ion batteries: a case of Ni0.8Co0.1Mn0.1O1.1 and LiNi0.8Co0.1Mn0.1O2. J Power Sources 342:495–503. https://doi.org/10.1016/j.jpowsour.2016.12.095
Wang J, Liu Z, Yan G, Li H, Peng W, Li X, Song L, Shih K (2016) Improving the electrochemical performance of lithium vanadium fluorophosphate cathode material: focus on interfacial stability. J Power Sources 329:553–557. https://doi.org/10.1016/j.jpowsour.2016.08.131
Yan G, Li X, Wang Z, Guo H, **ong X (2014) Beneficial effects of 1-propylphosphonic acid cyclic anhydride as an electrolyte additive on the electrochemical properties of LiNi0.5Mn1.5O4 cathode material. J Power Sources 263:231–238. https://doi.org/10.1016/j.jpowsour.2014.04.060
Li B, Shao R, Yan H, An L, Zhang B, Wei H, Ma J, **a D, Han X (2016) Understanding the stability for Li-rich layered oxide Li2RuO3 cathode. Adv Funct Mater 26(9):1330–1337. https://doi.org/10.1002/adfm.201504836
Koga H, Croguennec L, Ménétrier M, Mannessiez P, Weill F, Delmas C (2013) Different oxygen redox participation for bulk and surface: a possible global explanation for the cycling mechanism of Li1.20Mn0.54Co0.13Ni0.13O2. J Power Sources 236:250–258. https://doi.org/10.1016/j.jpowsour.2013.02.075
Koga H, Croguennec L, Menetrier M, Douhil K, Belin S, Bourgeois L, Suard E, Weill F, Delmas C (2013) Reversible oxygen participation to the redox processes revealed for Li1.20Mn0.54Co0.13Ni0.13O2. J Electrochem Soc 160(6):A786–A792. https://doi.org/10.1149/2.038306jes
Zeng J, Cui Y, Qu D, Zhang Q, Wu J, Zhu X, Li Z, Zhang X (2016) Facile synthesis of platelike hierarchical Li1.20Mn0.54Co0.13Ni0.13O2 with exposed {010} planes for high-rate and long cycling-stable lithium ion batteries. Acs Appl Mater Inter 8(39):26082–26090. https://doi.org/10.1021/acsami.6b08835
Kim S, Cho W, Zhang X, Oshima Y, Choi JW (2016) A stable lithium-rich surface structure for lithium-rich layered cathode materials. Nat Commun 7:13598–13606. https://doi.org/10.1038/ncomms13598
Gao K, Zhao SX, Guo S-T, Nan CW (2016) Improving rate capacity and cycling performance of lithium-rich high-Mn Li1.8[Mn0.7Co0.15Ni0.15]O2.675 cathode materials by Li2SiO3 coating. Electrochim Acta 206:1–9. https://doi.org/10.1016/j.electacta.2016.04.085
Chen H, Hu Q, Huang Z, He Z, Wang Z, Guo H, Li X (2016) Synthesis and electrochemical study of Zr-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode material for Li-ion battery. Ceram Int 42(1):263–269. https://doi.org/10.1016/j.ceramint.2015.08.104
Huang Z, Li X, Liang Y, He Z, Chen H, Wang Z, Guo H (2015) Structural and electrochemical characterization of Mg-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium ion batteries. Solid State Ionics 282:88–94. https://doi.org/10.1016/j.ssi.2015.10.005
Li Q, Li G, Fu C, Luo D, Fan J, Li L (2014) K+-doped Li1.2Mn0.54Ni0.13Co0.13O2: a novel cathode material with an enhanced cycling stability for lithium-ion batteries. Acs Appl Mater Inter 6(13):10330–10341. https://doi.org/10.1021/am5017649
Tang T, Zhang HL (2016) Synthesis and electrochemical performance of lithium-rich cathode material Li[Li0.2Ni0.15Mn0.55Co0.1-xAlx]O2. Electrochim Acta 191:263–269. https://doi.org/10.1016/j.electacta.2016.01.066
Li H, Fan LZ (2013) Effects of fluorine substitution on the electrochemical performance of layered Li-excess nickel manganese oxides cathode materials for lithium-ion batteries. Electrochim Acta 113:407–411. https://doi.org/10.1016/j.electacta.2013.09.135
Zheng J, Wu X, Yang Y (2013) Improved electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material by fluorine incorporation. Electrochim Acta 105:200–208. https://doi.org/10.1016/j.electacta.2013.04.150
Song JH, Kapylou A, Choi HS, BY Y, Matulevich E, Kang SH (2016) Suppression of irreversible capacity loss in Li-rich layered oxide by fluorine do**. J Power Sources 313:65–72. https://doi.org/10.1016/j.jpowsour.2016.02.058
Zheng J, Gu M, **ao J, Polzin BJ, Yan P, Chen X, Wang C, Zhang J (2014) Functioning mechanism of AlF3 coating on the Li- and Mn-rich cathode materials. Chem Mater 26(22):6320–6327. https://doi.org/10.1021/cm502071h
Liu X, Huang T, Yu A (2015) Surface phase transformation and CaF2 coating for enhanced electrochemical performance of Li-rich Mn-based cathodes. Electrochim Acta 163:82–92. https://doi.org/10.1016/j.electacta.2015.02.155
Huang B, Li X, Wang Z, Guo H, Shen L, Wang J (2014) A comprehensive study on electrochemical performance of Mn-surface-modified LNi0.8Co0.15Al0.05O2 synthesized by an in situ oxidizing-coating method. J Power Sources 252:200–207. https://doi.org/10.1016/j.jpowsour.2013.11.092
Liu Z, Peng W, Shih K, Wang J, Wang Z, Guo H, Yan G, Li X, Song L (2016) A MoS2 coating strategy to improve the comprehensive electrochemical performance of LiVPO4F. J Power Sources 315:294–301. https://doi.org/10.1016/j.jpowsour.2016.02.083
Liu Y, Zhang Z, Fu Y, Wang Q, Pan J, Su M, Battaglia VS (2016) Investigation the electrochemical performance of Li1.2Ni0.2Mn0.6O2 cathode material with ZnAl2O4 coating for lithium ion batteries. J Alloy Compd 685:523–532. https://doi.org/10.1016/j.jallcom.2016.05.329
**ong X, Wang Z, Yan G, Guo H, Li X (2014) Role of V2O5 coating on LiNiO2-based materials for lithium ion battery. J Power Sources 245:183–193. https://doi.org/10.1016/j.jpowsour.2013.06.133
Li L, Chen Z, Zhang Q, Xu M, Zhou X, Zhu H, Zhang K (2015) A hydrolysis-hydrothermal route for the synthesis of ultrathin LiAlO2-inlaid LiNi0.5Co0.2Mn0.3O2 as a high-performance cathode material for lithium ion batteries. J Mater Chem A 3(2):894–904. https://doi.org/10.1039/C4TA05902F
Zhang L, Li N, Wu B, Xu H, Wang L, Yang X-Q, Wu F (2014) Sphere-shaped hierarchical cathode with enhanced growth of nanocrystal planes for high-rate and cycling-stable Li-ion batteries. Nano Lett 15:656–661. https://doi.org/10.1021/nl5041594
Kim-Lohsoontorn P, Tiyapongpattana V, Asarasri N, Seeharaj P, Laosiripojana N (2014) Preparation of CeO2 nano rods through a sonication-assisted precipitation. Int J Appl Ceram Tec 11(4):645–653. https://doi.org/10.1111/ijac.12264
Daza L, Rangel CM, Baranda J, Casais MT, Martinez MJ, Alonso JA (2000) Modified nickel oxides as cathode materials for MCFC. J Power Sources 86(1-2):329–333. https://doi.org/10.1016/S0378-7753(99)00499-1
Liu K, Yang G-L, Dong Y, Shi T, Chen L (2015) Enhanced cycling stability and rate performance of Li[Ni0.5Co0.2Mn0.3]O2 by CeO2 coating at high cut-off voltage. J Power Sources 281:370–377. https://doi.org/10.1016/j.jpowsour.2014.12.131
Ha H-W, Jeong KH, Yun NJ, Hong MZ, Kim K (2005) Effects of surface modification on the cycling stability of LiNi0.8Co0.2O2 electrodes by CeO2 coating. Electrochim Acta 50(18):3764–3769. https://doi.org/10.1016/j.electacta.2005.01.022
Ha H-W, Yun NJ, Kim K (2007) Improvement of electrochemical stability of LiMn2O4 by CeO2 coating for lithium-ion batteries. Electrochim Acta 52(9):3236–3241. https://doi.org/10.1016/j.electacta.2006.09.066
Michalska M, Hamankiewicz B, Ziółkowska D, Krajewski M, Lipińska L, Andrzejczuk M, Czerwiński A (2014) Influence of LiMn2O4 modification with CeO2 on electrode performance. Electrochim Acta 136:286–291. https://doi.org/10.1016/j.electacta.2014.05.108
Zeng JH, Wang YF, Yang Y, Zhang J (2010) Synthesis of sea-urchin shaped γ-MnO2 nanostructures and their application in lithium batteries. J Mater Chem 20(48):10915–10918. https://doi.org/10.1039/c0jm01711f
Phadke S, Anouti M (2017) Effect of lithium salt concentration on the capacity retention of lithium rich NMC cathodes. Electrochim Acta 223:31–38. https://doi.org/10.1016/j.electacta.2016.12.010
Tran N, Croguennec L, Ménétrier M, Weill F, Biensan P, Jordy C, Delmas C (2008) Mechanisms associated with the “plateau” observed at high voltage for the over lithiated Li1.12(Ni0.425Mn0.425Co0.15)0.88O2 system. Chem Mater 20(15):4815–4825. https://doi.org/10.1021/cm070435m
Zheng J, Myeong S, Cho W, Yan P, **ao J, Wang C, Cho J, Zhang JG (2016) Li- and Mn-rich cathode materials: Challenges to commercialization. Adv Energy Mater 7:1601284–1601309. http://doi.org/10.1002/aenm.201601284
Sathiya M, Abakumov AM, Foix D, Rousse G, Ramesha K, Saubanère M, Doublet ML, Vezin H, Laisa CP, Prakash AS, Gonbeau D, VanTendeloo G, Tarascon JM (2015) Origin of voltage decay in high-capacity layered oxide electrodes. Nat Mater 14(2):230–238. https://doi.org/10.1038/nmat4137
Peralta D, Colin J-F, Boulineau A, Simonin L, Fabre F, Bouvet J, Feydi P, Chakir M, Chapuis M, Patoux S (2015) Role of the composition of lithium-rich layered oxide materials on the voltage decay. J Power Sources 280:687–694. https://doi.org/10.1016/j.jpowsour.2015.01.146
Lu C, Yang SQ, Wu H, Zhang Y, Yang XJ, Liang TH (2016) Enhanced electrochemical performance of Li-rich Li1.2Mn0.52Co0.08Ni0.2O2 cathode materials for Li-ion batteries by vanadium do**. Electrochim Acta 209:448–455. https://doi.org/10.1016/j.electacta.2016.05.119
Qing RP, Shi JL, Zhai YB, Zhang XD, Yin YX, Gu L, Guo YG (2016) Synthesis and electrochemical properties of a high capacity Li-rich cathode material in molten KCl-Na2CO3 flux. Electrochim Acta 196:749–755. https://doi.org/10.1016/j.electacta.2016.02.149
Nayak PK, Grinblat J, Levi E, Markovsky B, Aurbach D (2016) Effect of cycling conditions on the electrochemical performance of high capacity Li and Mn-rich cathodes for Li-ion batteries. J Power Sources 318:9–17. https://doi.org/10.1016/j.jpowsour.2016.03.107
Funding
This work was financially supported by the National Basic Research Program of China (973 Program. Grant No. 2014CB643406) and the National Science and Technology Support Program of China (No. 2015BAB06B00).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhou, LJ., Yin, ZL., Ding, ZY. et al. CeO2 coating to improve the performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 . Ionics 24, 2533–2542 (2018). https://doi.org/10.1007/s11581-017-2387-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2387-0