Abstract
Purpose
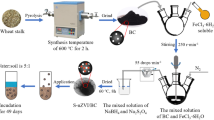
This research investigates the capability of biochar (BC)–supported nanoscale zero-valent iron (nZVI) composite (nZVI/BC) with strong adsorption and reduction properties for immobilization of hexavalent chromium (Cr(VI)) in Cr(VI)-polluted soil.
Methods
Liquid phase reduction method was used to prepare nZVI and nZVI/BC. Cr-contaminated soils were amended with BC, nZVI, and nZVI/BC. Toxicity characteristic leaching procedure (TCLP)–leachable Cr(Cr(VI)) was measured to evaluate the immobilization efficiency by three materials. The sequential extraction procedure (SEP) method was applied for Cr fraction analysis. The changes of soil properties and the reaction mechanism between Cr(VI) and nZVI/BC were also analyzed.
Results and discussion
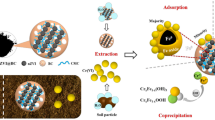
NZVI/BC exhibited a superior capacity for reducing TCLP-leachable Cr(VI) than nZVI and BC. The fraction analysis suggested that nZVI/BC could effectively reduce the toxicity of Cr(VI) by promoting the transformation of more accessible forms (exchangeable (EX)) into less accessible forms (iron-manganese oxides-bound (OX)). Compared with nZVI, the addition of BC and nZVI/BC could improve soil properties during a short term. X-Ray photoelectron spectroscopy (XPS) analysis showed that the redox reaction might be the main reaction mechanism between Cr(VI) and nZVI/BC.
Conclusion
NZVI/BC exhibited superior remediation capacity for Cr(VI)-polluted soil due to its high reduction and adsorption capacity. Moreover, those nano-particles could be recovered by magnetic separation after remediation process, and the recovery rate could reach more than 60%. Hence, nZVI/BC had a valuable utilization for the immobilization of Cr(VI) in soil.








Similar content being viewed by others
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly due to privacy.
References
Ahmed MMM, Balal Y, Ubaid AM, Chen D, Qumber A, Muhammad A, **aoe Y (2021) In situ synthesis of micro-plastics embedded sewage-sludge co-pyrolyzed biochar: implications for the remediation of Cr and Pb availability and enzymatic activities from the contaminated soil. J Clean Prod 302
Amezquita-Garcia HJ, Razo-Flores E, Cervantes FJ, Rangel-Mendez JR (2013) Activated carbon fibers as redox mediators for the increased reduction of nitroaromatics. Carbon 55:276–284
Bae S, Gim S, Kim H, Hanna K (2016) Effect of NaBH 4 on properties of nanoscale zero-valent iron and its catalytic activity for reduction of p-nitrophenol. Appl Catal B 182:541–549
Baragaño D, Alonso J, Gallego JR, Lobo MC, Gil-Díaz M (2020) Zero valent iron and goethite nanoparticles as new promising remediation techniques for As-polluted soils. Chemosphere 238
Bing Z, **nyang X, Fanqiang Z, Haibo L, ** C (2018) The hierarchical porous structure bio-char assessments produced by co-pyrolysis of municipal sewage sludge and hazelnut shell and Cu(II) adsorption kinetics. Environ Sci Pollut Res 25:19423–19435
Devi P, Saroha AK (2014) Synthesis of the magnetic biochar composites for use as an adsorbent for the removal of pentachlorophenol from the effluent. Bioresour Technol 169:525–531
Fu F, Ma J, **e L, Tang B, Han W, Lin S (2013) Chromium removal using resin supported nanoscale zero-valent iron. J Environ Manage 128:822–827
Geng B, ** Z, Li T, Qi X (2009) Preparation of chitosan-stabilized Fe 0 nanoparticles for removal of hexavalent chromium in water. Sci Total Environ 407:4994–5000
Geng H-X, Wang L (2019) Cadmium: toxic effects on placental and embryonic development. Environ Toxicol Pharmacol 67:102–107
Gil-Díaz M, Diez-Pascual S, González A, Alonso J, Rodríguez-Valdés E, Gallego JR, Lobo MC (2016) A nanoremediation strategy for the recovery of an As-polluted soil. Chemosphere 149:137–145
Herath I, Iqbal MCM, Al-Wabel MI, Abduljabbar A, Ahmad M, Usman ARA, Ok YS, Vithanage M (2017) Bioenergy-derived waste biochar for reducing mobility, bioavailability, and phytotoxicity of chromium in anthropized tannery soil. J Soils Sediments 17:731–740
Kotaś J, Stasicka Z (2000) Chromium occurrence in the environment and methods of its speciation. Environ Pollut 107:263–283
Li XQ, Zhang WX (2007) Sequestration of metal cations with zerovalent iron nanoparticles – a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J Phys Chem C 111:6939–6946
Liang B, Lehmann J, Sohi SP, Thies JE, O’Neill B, Trujillo L, Gaunt J, Solomon D, Grossman J, Neves EG, Luizão FJ (2009) Black carbon affects the cycling of non-black carbon in soil. Org Geochem 41:206–213
Liu T, Rao P, Lo IMC (2009) Influences of humic acid, bicarbonate and calcium on Cr(VI) reductive removal by zero-valent iron. Sci Total Environ 407:3407–3414
Liu X, Zhang S, Zhang X, Guo H, Lou Z, Zhang W, Chen Z (2022) Cr(VI) immobilization in soil using lignin hydrogel supported nZVI: immobilization mechanisms and long-term simulation. Chemosphere 305
Lu Y, **e Q, Tang L, Yu J, Wang J, Yang Z, Fan C, Zhang S (2021) The reduction of nitrobenzene by extracellular electron transfer facilitated by Fe-bearing biochar derived from sewage sludge. J Hazard Mater 403
Lv X, Xu J, Jiang G, Tang J, Xu X (2012) Highly active nanoscale zero-valent iron (nZVI)–Fe 3 O 4 nanocomposites for the removal of chromium(VI) from aqueous solutions. J Colloid Interface Sci 369:460–469
Lyu H, Tang J, Huang Y, Gai L, Zeng EY, Liber K, Gong Y (2017) Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem Eng J 322:516–524
Lyu H, Zhao H, Tang J, Gong Y, Huang Y, Wu Q, Gao B (2018) Immobilization of hexavalent chromium in contaminated soils using biochar supported nanoscale iron sulfide composite. Chemosphere 194:360–369
Manning BA, Kiser JR, Kwon H, Kanel SR (2007) Spectroscopic investigation of Cr(III)- and Cr(VI)-treated nanoscale zerovalent iron. Environ Sci Technol 41:586–592
Megharaj M, Avudainayagam S, Naidu R (2003) Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47:51–54
Miao C, Jian Z, Yaozong C, Peng H, Fang C, Xu W, **ye L, Chunyao G, Dongli H, Ke Z, Min G, Jianyu Z (2021) An efficient, economical, and easy mass production biochar supported zero˗valent iron composite derived from direct˗reduction natural goethite for Cu(II) and Cr(VI) remove. Chemosphere 285:131539–131539
Peng C, Zhai Y, Zhu Y, Xu B, Wang T, Li C, Zeng G (2016) Production of char from sewage sludge employing hydrothermal carbonization: char properties, combustion behavior and thermal characteristics. Fuel 176:110–118
Randolph P, Bansode RR, Hassan OA, Rehrah D, Ravella R, Reddy MR, Watts DW, Novak JM, Ahmedna M (2017) Effect of biochars produced from solid organic municipal waste on soil quality parameters. J Environ Manage 192:271–280
Ruili G, Hongqing H, Qingling F, Zhenhua L, Zhiqiang X, Umeed A, Jun Z, Yonghong L (2020) Remediation of Pb, Cd, and Cu contaminated soil by co-pyrolysis biochar derived from rape straw and orthophosphate: speciation transformation, risk evaluation and mechanism inquiry. Sci Total Environ 730
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Rehim A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Shang J, Zong M, Yu Y, Kong X, Du Q, Liao Q (2017) Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar. J Environ Manage 197
Shi L-n, Lin Y-M, Zhang X, Chen Z-l (2011) Synthesis, characterization and kinetics of bentonite supported nZVI for the removal of Cr(VI) from aqueous solution. Chem Eng J 171:708–715
Singh R, Misra V, Singh RP (2011) Synthesis, characterization and role of zero-valent iron nanoparticle in removal of hexavalent chromium from chromium-spiked soil. J Nanopart Res 13:4063–4073
Sneath HE, Hutchings TR, Leij FAAMd (2013) Assessment of biochar and iron filing amendments for the remediation of a metal, arsenic and phenanthrene co-contaminated spoil. Environ Pollut 178:361–366
Su H, Fang Z, Tsang PE, Fang J, Zhao D (2016) Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environ Pollut 214:94–100
Tan X-f, Liu Y-g, Gu Y-l, Xu Y, Zeng G-m, Hu X-j, Liu S-b, Wang X, Liu S-m, Li J (2016) Biochar-based nano-composites for the decontamination of wastewater: a review. Bioresour Technol 212:318–333
Tu C, Wei J, Guan F, Liu Y, Sun Y, Luo Y (2020) Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ Int 137
Vasarevičius S, Danila V, Paliulis D (2019) Application of stabilized nano zero valent iron particles for immobilization of available Cd2+, Cu2+, Ni2+, and Pb2+ ions in soil. Int J Environ Res 13:465–474
Wang Y, Zheng K, Zhan W, Huang L, Liu Y, Li T, Yang Z, Liao Q, Chen R, Zhang C, Wang Z (2021) Highly effective stabilization of Cd and Cu in two different soils and improvement of soil properties by multiple-modified biochar. Ecotoxicol Environ Saf 207:111294–111294
Wang Z, Chen G, Wang X, Li S, Liu Y, Yang G (2020) Removal of hexavalent chromium by bentonite supported organosolv lignin-stabilized zero-valent iron nanoparticles from wastewater. J Clean Prod 267
** C, Guangjian F, Haibo L, Yinghua L, Ran Z, Yu H, **nyang X (2021) Nanoscale zero-valent iron particles supported on sludge-based biochar for the removal of chromium (VI) from aqueous system. Environ Sci Pollut Res 29:3853–3863
Xu X, Zhao B, Sun M, Chen X, Zhang M, Li H, Xu S (2017) Co-pyrolysis characteristics of municipal sewage sludge and hazelnut shell by TG-DTG-MS and residue analysis. Waste Manage 62:91–100
Xu Y, Zhao D (2007) Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Res 41(10):2101–2108
Xue W, Huang D, Zeng G, Wan J, Zhang C, Xu R, Cheng M, Deng R (2018) Nanoscale zero-valent iron coated with rhamnolipid as an effective stabilizer for immobilization of Cd and Pb in river sediments. J Hazard Mater 341:381–389
Yan W, Herzing AA, Kiely CJ, Zhang W-x (2010) Nanoscale zero-valent iron (nZVI): aspects of the core-shell structure and reactions with inorganic species in water. J Contam Hydrol 118:96–104
Yin X, Liu W, Ni J (2014) Removal of coexisting Cr(VI) and 4-chlorophenol through reduction and Fenton reaction in a single system. Chem Eng J 248:89–97
Zhou X, Lv B, Zhou Z, Li W, **g G (2015) Evaluation of highly active nanoscale zero-valent iron coupled with ultrasound for chromium(VI) removal. Chem Eng J 281:155–163
Funding
This work was supported by The National Key Research and Development Project Subject (ZX20200121).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by XZ, YL, and YL. The first draft of the manuscript was written by GF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: **long Wang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Fan, G., Zhu, X. et al. The remediation of hexavalent chromium-contaminated soil by nanoscale zero-valent iron supported on sludge-based biochar. J Soils Sediments 23, 1607–1616 (2023). https://doi.org/10.1007/s11368-023-03433-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03433-x