Abstract
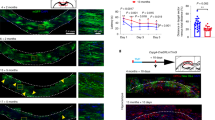
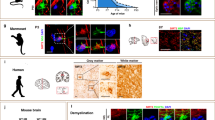
Age-associated cognitive decline is common among otherwise healthy elderly people, even in the absence of Alzheimer’s disease and neuron loss. Instead, white matter loss and myelin damage are strongly associated with cognitive decline. Myelin is subject to lifelong oxidative stress that damages the myelin sheath, which is repaired by cells of the oligodendrocyte lineage. This process is mediated by oligodendrocyte precursor cells (OPCs) that sense the damage and respond by proliferating locally and migrating to the region, where they differentiate into mature myelinating oligodendrocytes. In aging, extensive myelin damage, in combination with inefficient remyelination, leads to chronically damaged myelin and loss of efficient neuronal conduction. This study used the rhesus monkey model of normal aging to examine how myelin regeneration capacity is affected by age. Results show that older subjects have reduced numbers of new BCAS1 + myelinating oligodendrocytes, which are newly formed cells, and that this reduction is associated with poorer cognitive performance. Interestingly, this does not result from limited proliferation of progenitor OPCs. Instead, the transcription factor NKX2.2, which regulates OPCs differentiation, is significantly decreased in aged OPCs. This suggests that these OPCs have a diminished potential for differentiation into mature oligodendrocytes. In addition, mature oligodendrocytes have reduced RNA expression of two essential myelin protein markers, MBP and PLP. These data collectively suggest that in the normal aging brain, there is a reduction in regenerative OPCs as well as myelin production that impairs the capacity for remyelination.





Similar content being viewed by others
References
Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96.
Harada CN, Natelson MC, Love KL. Triebel, Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–52.
Freeman SH, et al. Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol. 2008;67(12):1205–12.
Guttmann CR, et al. White matter changes with normal aging. Neurology. 1998;50(4):972–8.
Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187–221.
Moss MB, et al. Recognition memory span in rhesus monkeys of advanced age. Neurobiol Aging. 1997;18(1):13–9.
Moss, M et al. Successful vs. unsuccessful aging in the rhesus monkey. In: Riddle DR, editor. Brain aging: models, methods, and mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 2.
Tigges J, et al. Survival rate and life span of rhesus monkeys at the Yerkes regional primate research center. Am J Primatol. 1988;15(3):263–73.
Wisco JJ, et al. An MRI study of age-related white and gray matter volume changes in the rhesus monkey. Neurobiol Aging. 2008;29(10):1563–75.
Bowley MP, et al. Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey. J Comp Neurol. 2010;518(15):3046–64.
Peters A, Rosene DL. In aging, is it gray or white? J Comp Neurol. 2003;462(2):139–43.
Salat DH, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:37–49.
Yoon B, et al. Region-specific changes of cerebral white matter during normal aging: a diffusion-tensor analysis. Arch Gerontol Geriatr. 2008;47(1):129–38.
Hill RA, Li AM, Grutzendler J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat Neurosci. 2018;21(5):683–95.
Lytle JM, et al. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia. 2009;57(3):270–85.
Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19(1):197–203.
Macchi M, et al. Mature oligodendrocytes bordering lesions limit demyelination and favor myelin repair via heparan sulfate production. Elife. 2020;9:e51735.
Lassmann H, van Horssen J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim Biophys Acta. 2016;1862(3):506–10.
Ljubisavljevic S. Oxidative Stress and Neurobiology of Demyelination. Mol Neurobiol. 2016;53(1):744–58.
Kohama SG, Rosene DL, Sherman LS. Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. Age (Dordr). 2012;34(5):1093–110.
Irvine KA, Blakemore WF. Age increases axon loss associated with primary demyelination in cuprizone-induced demyelination in C57BL/6 mice. J Neuroimmunol. 2006;175(1–2):69–76.
Sim FJ, et al. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22(7):2451–9.
Shen S, et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11(9):1024–34.
Safaiyan S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19(8):995–8.
Kotter MR, et al. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26(1):328–32.
Lampron A, et al. Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J Exp Med. 2015;212(4):481–95.
Soreq L, et al. Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep. 2017;18(2):557–70.
Woodruff RH, et al. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci. 2004;25(2):252–62.
Juurlink BH, Thorburne SK, Hertz L. Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia. 1998;22(4):371–8.
Neumann B, et al. Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells. Cell Stem Cell. 2019;25(4):473-485.e8.
Cantuti-Castelvetri L, et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. 2018;359(6376):684–8.
Natrajan MS, et al. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain. 2015;138(Pt 12):3581–97.
Hinks GL, Franklin RJ. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol Cell Neurosci. 2000;16(5):542–56.
Moore TL, et al. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol Aging. 2003;24(1):125–34.
Moore TL, et al. A non-human primate test of abstraction and set shifting: an automated adaptation of the Wisconsin Card Sorting Test. J Neurosci Methods. 2005;146(2):165–73.
Herndon JG, et al. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87(1):25–34.
Ngwenya LB, et al. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front Syst Neurosci. 2015;9:102.
Ngwenya LB, Peters A, Rosene DL. Light and electron microscopic immunohistochemical detection of bromodeoxyuridine-labeled cells in the brain: different fixation and processing protocols. J Histochem Cytochem. 2005;53(7):821–32.
Estrada LI, et al. Evaluation of long-term cryostorage of brain tissue sections for quantitative histochemistry. J Histochem Cytochem. 2017;65(3):153–71.
Giannaris EL, Rosene DL. A stereological study of the numbers of neurons and glia in the primary visual cortex across the lifespan of male and female rhesus monkeys. J Comp Neurol. 2012;520(15):3492–508.
Rosene DL, Roy NJ, Davis BJ. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem. 1986;34(10):1301–15.
Bauman MD, et al. Neuroprotective efficacy of P7C3 compounds in primate hippocampus. Transl Psychiatry. 2018;8(1):202.
West MJ. Basic stereology for biologists and neuroscientists. New York: Cold Spring Harbor Laboratory Press; 2012.
Fard MK, et al. BCAS1 expression defines a population of early myelinating oligodendrocytes in multiple sclerosis lesions. Sci Transl Med. 2017;9(419):eaam 7816.
Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4(3):407–10.
Nicaise AM, et al. Stem cells of the aging brain. Front Aging Neurosci. 2020;12:247.
Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science. 1982;218(4571):474–5.
Aberg ND, et al. Growth hormone and insuline-like growth factor-I and cellular regeneration in the adult brain. Cambridge: Academic Press Elsevier; 2005. p. 125–46.
Yeung MS, et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159(4):766–74.
Doucette JR, Jiao R, Nazarali AJ. Age-related and cuprizone-induced changes in myelin and transcription factor gene expression and in oligodendrocyte cell densities in the rostral corpus callosum of mice. Cell Mol Neurobiol. 2010;30(4):607–29.
Fancy SP, et al. Parallel states of pathological Wnt signaling in neonatal brain injury and colon cancer. Nat Neurosci. 2014;17(4):506–12.
Kondo T, Raff M. The Id4 HLH protein and the timing of oligodendrocyte differentiation. EMBO J. 2000;19(9):1998–2007.
Liu A, et al. A molecular insight of Hes5-dependent inhibition of myelin gene expression: old partners and new players. EMBO J. 2006;25(20):4833–42.
Wang S, et al. A role for the helix-loop-helix protein Id2 in the control of oligodendrocyte development. Neuron. 2001;29(3):603–14.
Lu QR, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109(1):75–86.
Sugimori M, et al. Ascl1 is required for oligodendrocyte development in the spinal cord. Development. 2008;135(7):1271–81.
Soula C, et al. Distinct sites of origin of oligodendrocytes and somatic motoneurons in the chick spinal cord: oligodendrocytes arise from Nkx2.2-expressing progenitors by a Shh-dependent mechanism. Development. 2001;128(8):1369–79.
Fu H, et al. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development. 2002;129(3):681–93.
Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31(5):791–807.
Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330(6005):779–82.
Zhang C, et al. The transcription factor NKX2-2 regulates oligodendrocyte differentiation through domain-specific interactions with transcriptional corepressors. J Biol Chem. 2020;295(7):1879–88.
Qi Y, et al. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128(14):2723–33.
Zhu Q, et al. Genetic evidence that Nkx2.2 and Pdgfra are major determinants of the timing of oligodendrocyte differentiation in the develo** CNS. Development. 2014;141(3):548–55.
Pringle N, et al. PDGF A chain homodimers drive proliferation of bipotential (O-2A) glial progenitor cells in the develo** rat optic nerve. EMBO J. 1989;8(4):1049–56.
**e F, et al. Age-related decline of myelin proteins is highly correlated with activation of astrocytes and microglia in the rat CNS. Int J Mol Med. 2013;32(5):1021–8.
Ahn JH, et al. Age-dependent differences in myelin basic protein expression in the hippocampus of young, adult and aged gerbils. Lab Anim Res. 2017;33(3):237–43.
Jahn O, Tenzer S, Werner HB. Myelin proteomics: molecular anatomy of an insulating sheath. Mol Neurobiol. 2009;40(1):55–72.
Spitzer SO, et al. Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron. 2019;101(3):459-471.e5.
Nicaise AM, et al. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc Natl Acad Sci U S A. 2019;116(18):9030–9.
Psachoulia K, et al. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5(3–4):57–67.
Young KM, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77(5):873–85.
Lee S, et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods. 2012;9(9):917–22.
Goebbels S, et al. A neuronal PI(3,4,5)P. Nat Neurosci. 2017;20(1):10–5.
Feldman ML, Peters A. Ballooning of myelin sheaths in normally aged macaques. J Neurocytol. 1998;27(8):605–14.
Wosik K, et al. Oligodendrocyte injury in multiple sclerosis: a role for p53. J Neurochem. 2003;85(3):635–44.
Jurewicz A, et al. Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain. 2005;128(Pt 11):2675–88.
Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61(5):471–9.
Horner PJ, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20(6):2218–28.
Dimou L, et al. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28(41):10434–42.
Hughes EG, et al. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16(6):668–76.
Bubb EJ, Metzler-Baddeley C, Aggleton JP. The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev. 2018;92:104–27.
Siffredi V, et al. Neural correlates of working memory in children and adolescents with agenesis of the corpus callosum: An fMRI study. Neuropsychologia. 2017;106:71–82.
Treble A, et al. Working memory and corpus callosum microstructural integrity after pediatric traumatic brain injury: a diffusion tensor tractography study. J Neurotrauma. 2013;30(19):1609–19.
Koutsoudaki PN, et al. Cellular senescence and failure of myelin repair in multiple sclerosis. Mech Ageing Dev. 2020;192:111366.
Ruckh JM, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10(1):96–103.
Green AJ, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481–9.
Wang H, et al. Quetiapine Inhibits microglial activation by neutralizing abnormal STIM1-mediated intercellular calcium homeostasis and promotes myelin repair in a cuprizone-induced mouse model of demyelination. Front Cell Neurosci. 2015;9:492.
Lloyd AF, Miron VE. The pro-remyelination properties of microglia in the central nervous system. Nat Rev Neurol. 2019;15(8):447–58.
Acknowledgements
We are grateful for the invaluable technical assistance of Karen Slater, Penny Shultz, Alejandra Avendano, Brady Hirshfeld, and Bryce Conner.
Funding
This research was supported by NIH/NIA Grants: 1RF1AG062831-01, 2RF1AG043640-06.
Author information
Authors and Affiliations
Contributions
Christina Dimovasili: Conceptualization, Methodology, Investigation, Analysis, Original draft preparation. Ashley Fair: Visualization, Investigation. Isabella Garza: Visualization, Investigation. Katelyn Batterman: Methodology, Review, and Editing. Farzad Mortazavi: Data curation, Review, and Editing. Tara Moore: Methodology, Resources. Douglas Rosene: Review and Editing, Funding, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Dimovasili, C., Fair, A.E., Garza, I.R. et al. Aging compromises oligodendrocyte precursor cell maturation and efficient remyelination in the monkey brain. GeroScience 45, 249–264 (2023). https://doi.org/10.1007/s11357-022-00621-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00621-4