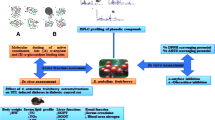
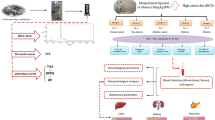
Abstract
In the recent past, phytomolecules are exponentially applied in discovering the antidiabetic drug due to less adverse effects. This work screened the active solvent fraction of Lespedeza cuneata based on the phytochemical, enzyme inhibition, and antioxidant properties. The antioxidant efficacy of the different fractions of the L. cuneata was assessed by 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ferric reducing power, hydrogen peroxide, and hydroxyl radical scavenging assays. The digestive enzyme (α-amylase and α-glucosidase) inhibitory activity was also evaluated. The phytochemical composition of ethyl acetate fraction of L. cuneata (Lc-EAF) was studied by UHPLC-QTOF–MS/MS. The effect of Lc-EAF treatments on glucose uptake was studied in insulin resistance HepG2 cells (IR-HepG2). Further, the antidiabetic effect of Lc-EAF in streptozotocin (STZ)-induced diabetic mice were demonstrated. Ethyl acetate, hexane, and methanol fractions of the L. cuneata showed notable antioxidant, α-amylase, and α-glucosidase inhibitory properties. Among the fractions, Lc-EAF was found to be the most potent. The Lc-EAF exhibited an IC50 of 205.32 ± 23.47 µg/mL and 105.32 ± 13.93 µg/mL for α-amylase and α-glucosidase inhibition, respectively. In addition, 75 µg/mL of Lc-EAF exposure enhanced glucose uptake (68.23%) in IR-HepG2 cells. In vivo study indicated that treatment of Lc-EAF (100 mg/kg b.wt) maintained the blood glucose level through reduced insulin level while improving the lipid profile, hepatic, and renal markers. These findings suggest that Lc-EAF could be considered a prominent source for antidiabetic, anti-hyperlipidemic, and anti-ROS potentials.







Similar content being viewed by others
Data availability
The data are available from the corresponding author upon reasonable request and with permission of the study sponsor.
Abbreviations
- ABTS:
-
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- ALT:
-
Aspartate aminotransferase
- B.wt:
-
Body weight
- BUN:
-
Blood urea nitrogen
- DM:
-
Diabetes mellitus
- DNS:
-
3, 5-Dinitrosalicylic acid
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- GAE:
-
Gallic acid equivalents
- HDL-C:
-
High-density lipoprotein cholesterol
- IP:
-
Intraperitoneal
- IR-HepG2:
-
Insulin resistance HepG2 cells
- Lc-EAF:
-
Ethyl acetate fractions of L. cuneata
- Lc-HF:
-
Hexane fractions of L. cuneata
- Lc-MF:
-
Methanol fractions of L. cuneata
- LDL-C:
-
Low-density lipoprotein cholesterol
- QE:
-
Quercetin equivalents
- STZ:
-
Streptozotocin
- TPC:
-
Total phenolic content
References
Ali MK, Seiglie JA, Narayan KMV (2021) Progress in diabetes prevention or epidemiology—or both, or neither? Lancet Diabetes Endocrinol 9:190
Anand Mariadoss AV, Krishnan Dhanabalan A, Munusamy H, Gunasekaran K, David E (2018) In silico studies towards enhancing the anticancer activity of phytochemical phloretin against cancer drug targets. Current Drug Therapy 13:174–188
Bender O, Llorent-Martínez EJ, Zengin G, Mollica A, Ceylan R, Molina-García L, Fernández-de Córdova ML, Atalay A (2018) Integration of in vitro and in silico perspectives to explain chemical characterization, biological potential and anticancer effects of Hypericum salsugineum: a pharmacologically active source for functional drug formulations. PLoS ONE 13:e0197815
Bianco A, Buiarelli F, Cartoni G, Coccioli F, Muzzalupo I, Polidori A, Uccella N (2001) Analysis by HPLC-MS/MS of biophenolic components in olives and oils. Anal Lett 34:1033–1051
Cantley J, Ashcroft FM (2015) Q&A: insulin secretion and type 2 diabetes: why do β-cells fail? BMC Biol 13:33–33
Cho EJ, Lee SG, Kim DO (2009) The effect of Lespedeza cuneata extract for antioxidative and whitening effect. J Life Resour Sci Res 28:34–38
Dineshkumar B, Mitra A, Manjunatha M (2010) A comparative study of alpha amylase inhibitory activities of common anti-diabetic plants at Kharagpur 1 block. Int J Green Pharm 4:115–121
Fernandes de Oliveira AM, Sousa Pinheiro L, Souto Pereira CK, Neves Matias W, Albuquerque Gomes R, Souza Chaves O, de Souza V, MdF N, de Almeida R, Simões de Assis T (2012) Total phenolic content and antioxidant activity of some Malvaceae family species. Antioxidants (basel, Switzerland) 1:33–43
Geng P, Sun J, Zhang M, Li X, Harnly JM, Chen P (2016) Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMS(n) and mass defect filtering. J Mass Spectrom: JMS 51:914–930
Guan L, Yang H, Cai Y, Sun L, Di P, Li W, Liu G, Tang Y (2019) ADMET-score–a comprehensive scoring function for evaluation of chemical drug-likeness. MedChemComm 10:148–157
Halliwell B, Gutteridge JMC, Aruoma OI (1987) The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165:215–219
Honda M, Hara Y (1993) Inhibition of rat small intestinal sucrase and. ALPHA.-glucosidase activities by tea polyphenols. Biosci Biotechnol Biochem 57:123–124
Huneif MA, Alqahtani SM, Abdulwahab A, Almedhesh SA, Mahnashi MH, Riaz M, Ur-Rahman N, Jan MS, Ullah F, Aasim M, Sadiq A (2022) α-glucosidase, α-amylase andantioxidant evaluations of isolated bioactives from wild strawberry. Molecules 27:3444
Jeong MS, Park S, Han EJ, Park SY, Kim MJ, Jung K, Cho S-H, Kim S-Y, Yoon W-J, Ahn G, Kim K-N (2020) Pinus thunbergii PARL leaf protects against alcohol-induced liver disease by enhancing antioxidant defense mechanism in BALB/c mice. J Funct Foods 73:104116
Karar M, Kuhnert N (2015) UPLC-ESI-Q-TOF-MS/MS Characterization of phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) leaves, fruits and their herbal derived drops (Crataegutt Tropfen). J Chem Biol Ther 1:102
Kaszás L, Alshaal T, El-Ramady H, Kovács Z, Koroknai J, Elhawat N, Nagy É, Cziáky Z, Fári M, Domokos-Szabolcsy É (2020) Identification of bioactive phytochemicals in leaf protein concentrate of Jerusalem artichoke (Helianthus tuberosus L). Plants 9:889
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J (2020) Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Global Health 10:107–111
Khurana N, Ishar MPS, Gajbhiye A, Goel RK (2011) PASS assisted prediction and pharmacological evaluation of novel nicotinic analogs for nootropic activity in mice. Eur J Pharmacol 662:22–30
Kifayatullah M, Mustafa MS, Sengupta P, Sarker MMR, Das A, Das SK (2015) Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr. in BALB/c mice. J Acute Dis 4:309–315
Kim S-J, Kim D-W (2007) Antioxidative activity of hot water and ethanol extracts of Lespedeza cuneata seeds. Korean J Food Preservation 14:332–335
Kim J-S, Kim M-J (2010) In vitro antioxidant activity of Lespedeza cuneata methanolic extracts. J Med Plants Res 4:674–679
Kim H-J, Jeon S-M, Lee M-K, Cho Y-Y, Kwon E-Y, Lee JH, Choi M-S (2010) Comparison of hesperetin and its metabolites for cholesterol-lowering and antioxidative efficacy in hypercholesterolemic hamsters. J Med Food 13:808–814
Kim SM, Kang K, Jho EH, Jung YJ, Nho CW, Um BH, Pan CH (2011) Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide. Phytother Res 25:1011–1017
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 2013:162750
Lee H-J, Lim G-N, Park M-A, Park S-N (2011) Antibacterial and antioxidative activity of Lespedeza cuneata G. Don Extracts Microbiol Biotechnol Lett 39:63–69
Lee H, Jung JY, Hwangbo M, Ku SK, Kim YW, Jee SY (2013) Anti-inflammatory effects of Lespedeza cuneata in vivo and in vitro. Korea J Herbol 28:83–92
Lee JS, Lee AY, Quilantang NG, Geraldino PJL, Cho EJ, Lee S (2019) Antioxidant activity of avicularin and isovitexin from Lespedeza cuneata. J Appl Biol Chem 62:143–147
Li R, Liu S-k, Song W, Wang Y, Li Y-j, Qiao X, Liang H, Ye M (2014) Chemical analysis of the Tibetan herbal medicine Carduus acanthoides by UPLC/DAD/qTOF-MS and simultaneous determination of nine major compounds. Anal Methods 6:7181–7189
Lipinski CA (2004) Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1:337–341
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23:3–25
Liu S, Yu Z, Zhu H, Zhang W, Chen Y (2016) In vitro α-glucosidase inhibitory activity of isolated fractions from water extract of Qingzhuan dark tea. BMC Complement Altern Med 16:378–378
Magliano DJ, Chen L, Islam RM, Carstensen B, Gregg EW, Pavkov ME, Andes LJ, Balicer R, Baviera M, Boersma-van Dam E (2021) Trends in the incidence of diagnosed diabetes: a multicountry analysis of aggregate data from 22 million diagnoses in high-income and middle-income settings. Lancet Diabetes Endocrinol 9:203–211
Malik NH, Zin ZM, Razak SBA, Ibrahim K, Zainol MK (2017) Antioxidative activities and flavonoids contents in leaves of selected mangrove species in Setiu wetlands extracted using different solvents. J Sustain Sci Manag 3:14–22
Mariadoss AVA, Saravanakumar K, Sathiyaseelan A, Wang M-H (2020) Preparation, characterization and anti-cancer activity of graphene oxide–silver nanocomposite. J Photochem Photobiol B 210:111984
Mariadoss AVA, Park S, Saravanakumar K, Sathiyaseelan A, Wang M-H (2021) Ethyl acetate fraction of Helianthus tuberosus L. induces anti-diabetic, and wound-healing activities in insulin-resistant human liver cancer and mouse fibroblast cells. Antioxidants 10:99
Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ (2014) Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes 5:444–470
Middleton E, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Mirzaei M, Rahmaninan M, Mirzaei M, Nadjarzadeh A (2020) Epidemiology of diabetes mellitus, pre-diabetes, undiagnosed and uncontrolled diabetes in Central Iran: results from Yazd health study. BMC Public Health 20:166
Mohan V, Khunti K, Chan SP, Fadlo Filho F, Tran NQ, Ramaiya K, Joshi S, Mithal A, Mbaye MN, Nicodemus NA (2020) Management of type 2 diabetes in develo** countries: balancing optimal glycaemic control and outcomes with affordability and accessibility to treatment. Diabetes Therapy 11:15–35
Nomura M, Takahashi T, Nagata N, Tsutsumi K, Kobayashi S, Akiba T, Yokogawa K, Moritani S, Miyamoto K-i (2008) Inhibitory mechanisms of flavonoids on insulin-stimulated glucose uptake in MC3T3-G2/PA6 adipose cells. Biol Pharm Bull 31:1403–1409
Oh S-H, Ku H, Park KS (2021) Prevalence and socioeconomic burden of diabetes mellitus in South Korean adults: a population-based study using administrative data. BMC Public Health 21:1–13
Ollerton RL, Playle R, Ahmed K, Dunstan FD, Luzio SD, Owens DR (1999) Day-to-day variability of fasting plasma glucose in newly diagnosed type 2 diabetic subjects. Diabetes Care 22:394–398
Oyaizu M (1986) Studies on products of browning reaction:antioxidative activities of product of browning reaction prepared from glucosamine. Japan J Nutr 44:307–315
Oyewande AA, Iqbal B, Abdalla LF, Karim F, Khan S (2020) An overview of the pathophysiology of metabolic changes and their sequence of occurrence in obese diabetic females: a narrative review. Cureus 12:e10947
Ozcan F, Ozmen A, Akkaya B, Aliciguzel Y, Aslan M (2012) Beneficial effect of myricetin on renal functions in streptozotocin-induced diabetes. Clin Exp Med 12:265–272
Park Y-I, Cha YE, Jang M, Park R, Namkoong S, Kwak J, Jang I-S, Park J (2020) The flower extract of Abelmoschus manihot (Linn.) increases cyclin d1 expression and activates cell proliferation. J Microbiol Biotechnol 30:1044–1050
Piraud M, Vianey-Saban C, Petritis K, Elfakir C, Steghens J-P, Morla A, Bouchu D (2003) ESI-MS/MS analysis of underivatised amino acids: a new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun Mass Spectrom 17:1297–1311
Poovitha S, Parani M (2016) In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L). BMC Complement Altern Med 16:185
Qin N, Chen Y, ** M-N, Zhang C, Qiao W, Yue X-L, Duan H-Q, Niu W-Y (2016) Anti-obesity and antidiabetic effects of flavonoid derivative (Fla-CN) via microRNA in high fat diet induced obesity mice. Eur J Pharm Sci 82:52–63
Rahmati M, Gharakhanlou R, Movahedin M, Mowla SJ, Khazani A, Fouladvand M, Golbar SJ (2015) Treadmill training modifies KIF5B motor protein in the STZ-induced diabetic rat spinal cord and sciatic nerve. Arch Iran Med 18:94–101
Röder PV, Wu B, Liu Y, Han W (2016) Pancreatic regulation of glucose homeostasis. Exp Mol Med 48:e219–e219
Rohn S, Rawel HM, Kroll J (2002) Inhibitory effects of plant phenols on the activity of selected enzymes. J Agric Food Chem 50:3566–3571
Ruan J, Yan J, Zheng D, Sun F, Wang J, Han L, Zhang Y, Wang T (2019) Comprehensive chemical profiling in the ethanol extract of Pluchea indica aerial parts by liquid chromatography/mass spectrometry analysis of its silica gel column chromatography fractions. Molecules (basel, Switzerland) 24:2784
Saadane A, Lessieur EM, Du Y, Liu H, Kern TS (2020) Successful induction of diabetes in mice demonstrates no gender difference in development of early diabetic retinopathy. PLoS ONE 15:e0238727–e0238727
Saravanakumar K, Sathiyaseelan A, Mariadoss AVA, Wang M-H (2021) Antioxidant and antidiabetic properties of biocompatible ceria oxide (CeO2) nanoparticles in mouse fibroblast NIH3T3 and insulin resistant HepG2 cells. Ceram Int 47:8618–8626
Sharma BR, Kim MS, Yokozawa T, Rhyu DY (2014) Antioxidant and antidiabetic activities of Lespedeza cuneata water extract. J Med Plants Res 8:935–941
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178
Srinivasan S, Pari L (2013) Antihyperlipidemic effect of diosmin: a citrus flavonoid on lipid metabolism in experimental diabetic rats. J Funct Foods 5:484–492
Sudha P, Zinjarde SS, Bhargava SY, Kumar AR (2011) Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement Altern Med 11:1–10
Sun C et al (2020) Dietary polyphenols as antidiabetic agents: advances and opportunities. Food Frontiers 1:18–44
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JC, Mbanya JCJDR (2022) IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119
Tshiyoyo KS, Bester MJ, Serem JC, Apostolides Z (2022) In-silico reverse docking and in-vitro studies identified curcumin, 18α-glycyrrhetinic acid, rosmarinic acid, and quercetin as inhibitors of α-glucosidase and pancreatic α-amylase and lipid accumulation in HepG2 cells, important type 2 diabetes targets. J Mol Str 1266:133492
Unuofin JO, Otunola GA, Afolayan AJ (2017) Phytochemical screening and in vitro evaluation of antioxidant and antimicrobial activities of Kedrostis africana (L.) Cogn. Asian Pac J Trop Biomed 7:901–908
Vinayagam R, Jayachandran M, Xu B (2016) Antidiabetic effects of simple phenolic acids: a comprehensive review. Phytother Res 30:184–199
Vinayagam R, **ao J, Xu B (2017) An insight into antidiabetic properties of dietary phytochemicals. Phytochem Rev 16:535–553
Virgen-Ortíz JJ, Ibarra-Junquera V, Escalante-Minakata P, Centeno-Leija S, Serrano-Posada H, de Jesús O-P, Pérez-Martínez JD, Osuna-Castro JA (2016) Identification and functional characterization of a fructooligosaccharides-forming enzyme from Aspergillus aculeatus. Appl Biochem Biotechnol 179:497–513
Wang Y, **ang L, Wang C, Tang C, He X (2013) Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 8:e71144–e71144
Wang Y, Wu S, Wen F, Cao Q (2020) Diabetes mellitus as a risk factor for retinal vein occlusion: a meta-analysis. Medicine 99:e19319
Yoo G, Park SJ, Lee TH, Yang H, Baek Y-s, Kim N, Kim YJ, Kim SH (2015) Flavonoids isolated from Lespedeza cuneata G. Don and their inhibitory effects on nitric oxide production in lipopolysaccharide-stimulated BV-2 microglia cells. Pharmacogn Mag 11:651
Zakaria Z, Aziz R, Lachimanan YL, Sreenivasan S, Rathinam X (2008) Antioxidant activity of Coleus blumei, Orthosiphon stamineus, Ocimum basilicum and Mentha arvensis from Lamiaceae family. Int J Nat Eng Sci 2:93–95
Zhang C, Zhou J, Yang J, Li C, Ma J, Zhang D, Zhang D (2016) Two new phenylpropanoid glycosides from the aerial parts of Lespedeza cuneata. Acta Pharmaceutica Sinica B 6:564–567
Zhang L, Tu Z-c, **e X, Wang H, Wang H, Wang Z-x, Sha X-m, Lu Y (2017) Jackfruit (Artocarpus heterophyllus Lam.) peel: a better source of antioxidants and a-glucosidase inhibitors than pulp, flake and seed, and phytochemical profile by HPLC-QTOF-MS/MS. Food Chem 234:303–313
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Zhou J, Zheng X, Yang Q, Liang Z, Li D, Yang X, Xu J (2013) Optimization of ultrasonic-assisted extraction and radical-scavenging capacity of phenols and flavonoids from Clerodendrum cyrtophyllum Turcz leaves. PLoS ONE 8:e68392
Zhou J, Li C-J, Yang J-Z, Ma J, Wu L-Q, Wang W-J, Zhang D-M (2016) Phenylpropanoid and lignan glycosides from the aerial parts of Lespedeza cuneata. Phytochemistry 121:58–64
Donato MT, Tolosa L, Gómez-Lechón MJ (2015) Culture and functional characterization of human hepatoma HepG2 cells, Protocols in In Vitro Hepatocyte Research. Springer, pp. 77–93
Funding
This study was supported by the National Research Foundation of Korea (NRF) (2019R1A1055452; 2021R1I1A1A01057742; 2022R1A2C2091029; 2022R1F1A1063364).
Author information
Authors and Affiliations
Contributions
Arokia Vijaya Anand Mariadoss: conceptualization, data curation, formal analysis, investigation, methodology, visualization, roles/writing-original draft, writing-review and editing. SeonJu Park: formal analysis, investigation. Kandasamy Saravanakumar: data curation, formal analysis, validation, review and editing. Anbazhagan Sathiyaseelan: software, formal analysis, data curation, validation. Myeong-Hyeon Wang: funding acquisition, project administration, resources, software, supervision, validation.
Corresponding author
Ethics declarations
Ethics approval
All authors hereby declare that “Principles of Laboratory Animal Care” (NIH publication No. 85–23, revised 1985) were followed, as well as specific national laws where applicable. All animal experiments were performed under a protocol approved by the Local Institutional Animal Ethics Committee of Kangwon National University, Republic of Korea.
Consent to participate
The authors agreed to participate in this work.
Consent for publication
The work in this manuscript has not been previously published and is not under consideration of other journals.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mariadoss, A.V.A., Park, S., Saravanakumar, K. et al. Phytochemical profiling, in vitro antioxidants, and antidiabetic efficacy of ethyl acetate fraction of Lespedeza cuneata on streptozotocin-induced diabetic rats. Environ Sci Pollut Res 30, 60976–60993 (2023). https://doi.org/10.1007/s11356-023-26412-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26412-8