Abstract
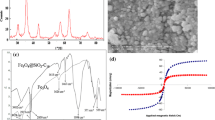
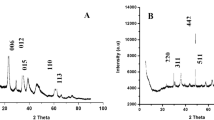
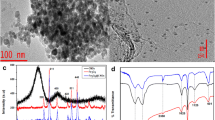
There exists a high demand for fast, simple, and reliable methodologies for determining the presence of organochlorine pesticides (OCPs) on environmental samples. Moreover, the toxicity and accumulation of potential OCPs in several environments have led to the development of technologies that achieve their removal from contaminated waters. In this study, a novel method combining a dispersive liquid–liquid microextraction procedure based on the solidification of floating organic drop is developed and validated for the extraction, preconcentration, and determination of 10 OCPs: α-BHC, p,p′-DDE, δ-BHC, dieldrin, p,p′-DDT, endosulfan I, endosulfan sulfate, heptachlor, heptachlor epoxide (isomer B), and methoxychlor in water samples. The results show that the calibration curves were linear for all the studied compounds, and the coefficients of correlation higher than 0.99. The variation coefficient for precision and accuracy was lower than 10%, and the accuracy ranged from 93 to 105%. Low limit of detection and limit of quantification values ranging from 0.06–3.00 ng mL−1 and 0.20–10 ng mL−1 were obtained, respectively. The capability of the proposed method was confirmed using an analysis of the water samples before and after the degradation process; this was achieved by employing nanomaterials, while performing an analysis of 160 real samples that were sourced from a Brazilian river. A cobalt-doped magnetite was applied for the environmental remediation of the studied compounds, and it was verified that the novel material has the potential to be used in environmental remediation with a degradation efficiency exceeding 80% for the majority of the studied compounds.







Similar content being viewed by others
References
Ali SN, Baqar M, Mumtaz M, Ashraf U, Anwar MN, Qadir A, Ahmad SR, Nizami AS, Jun H (2020) Organochlorine pesticides in the surrounding soils of POPs destruction facility: source fingerprinting, human health, and ecological risks assessment. Environ Sci Pollut Res 27:7328–7340
Aver GF, Espín S, Dal Corno RDB, García-Fernández AJ, Petry MV (2020) Organochlorine pesticides in feathers of three raptor species in southern Brazil. Environ Sci Pollut Res 27:5971–5980
Barreiro JC, Capelato MD, Martin-Neto L, Bruun Hansen HC (2007) Oxidative decomposition of atrazine by a Fenton-like reaction in a H2O2/ferrihydrite system. Water Res 41:55–62
Chormey DS, Fırat M, Bakırdere S (2018) Multivariate optimization of binary solvent microextraction for the simultaneous determination of endocrine disruptive phenolic compounds and organochlorine pesticides in wastewater and sludge samples by GC-MS. Water Air Soil Pollut 229
Cortada C, Vidal L, Pastor R, Santiago N, Canals A (2009) Determination of organochlorine pesticides in water samples by dispersive liquid–liquid microextraction coupled to gas chromatography–mass spectrometry. Anal Chim Acta 649:218–221
Costa RCC, Lelis MFF, Oliveira LCA et al (2003) Remarkable effect of co and Mn on the activity of Fe3−xMxO4 promoted oxidation of organic contaminants in aqueous medium with H2O2. Catal Commun 4:525–529
Costa RCC, Lelis MF, Oliveira LC et al (2006) Novel active heterogeneous Fenton system based on Fe3−xMxO4 (Fe, Co, Mn, Ni): the role of M2+ species on the reactivity towards H2O2 reactions. J Hazard Mater 129:171–178
Elliott DW, Lien H-L, Zhang W-X (2009) Degradation of lindane by zero-valent iron nanoparticles. J Environ Eng 135:317–324
Esteves LCR, Oliveira TR, Souza EC et al (2015) A fast and environment-friendly method for determination of chemical oxygen demand by using the heterogeneous Fenton-like process (H2O2/Fe3−xCoxO4 nanoparticles) as an oxidant. Talanta 135:75–80
Huang W, He Y, **ao J, Huang Y, Li A, He M, Wu K (2019) Risk of breast cancer and adipose tissue concentrations of polychlorinated biphenyls and organochlorine pesticides: a hospital-based case-control study in Chinese women. Environ Sci Pollut Res 26:32128–32136
Ikehata K, El-Din MG (2006) Aqueous pesticide degradation by hydrogen peroxide/ultraviolet irradiation and Fenton-type advanced oxidation processes: a review. J Environ Eng Sci 5:81–135
Joo SH, Zhao D (2008) Destruction of lindane and atrazine using stabilized iron nanoparticles under aerobic and anaerobic conditions: effects of catalyst and stabilizer. Chemosphere 70:418–425
Kocúrová L, Balogh IS, Šandrejová J, Andruch V (2012) Recent advances in dispersive liquid–liquid microextraction using organic solvents lighter than water. A review. Microchem J 102:11–17
Kwan WP, Voelker BM (2003) Rates of hydroxyl radical generation and organic compound oxidation in mineral-catalyzed Fenton-like systems. Environ Sci Technol 37:1150–1158
Leong MI, Huang SD (2008) Dispersive liquid–liquid microextraction method based on solidification of floating organic drop combined with gas chromatography with electron-capture or mass spectrometry detection. J Chromatogr A 1211:8–12
Li S, Lu C, Zhu F, Jiang R, Ouyang G (2015) Preparation of C18 composite solid phase microextraction fiber and its application to the determination of organochlorine pesticides in water samples. Anal Chim Acta 873:57–62
Lv X, Prastistho W, Yang Q, Tokoro C (2020) Application of nano-scale zero-valent iron adsorbed on magnetite nanoparticles for removal of carbon tetrachloride: products and degradation pathway. Appl Organomet Chem:34
Magalhães F, Pereira MC, Botrel SEC, Fabris JD, Macedo WA, Mendonça R, Lago RM, Oliveira LCA (2007) Cr-containing magnetites Fe3−xCrxO4: the role of Cr3+ and Fe2+ on the stability and reactivity towards H2O2 reactions. Appl Catal A 332:115–123
Mousavi L, Tamiji Z, Khoshayand MR (2018) Applications and opportunities of experimental design for the dispersive liquid-liquid microextraction method - a review. Talanta 190:335–356
Nasiri M, Ahmadzadeh H, Amiri A (2020) Sample preparation and extraction methods for pesticides in aquatic environments: A review. Trends Anal Chem 123:115772. https://doi.org/10.1016/j.trac.2019.115772
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater 98:33–50
Olisah C, Adeniji AO, Okoh OO, Okoh AI (2019) Occurrence and risk evaluation of organochlorine contaminants in surface water along the course of Swartkops and Sundays River Estuaries, Eastern Cape Province, South Africa. Environ Geochem Health 41:2777–2801
Paknikar KM, Nagpal V, Pethkar AV, Rajwade JM (2005) Degradation of lindane from aqueous solutions using iron sulfide nanoparticles stabilized by biopolymers. Sci Technol Adv Mater 6:370–374
Prates CB, Gebara SS, Ré-Poppi N (2011) Análise de pesticidas organoclorados em água usando a microextração em fase sólida por headspace com cromatografia gasosa e espectrometria de massas. Química Nova 34:1260–1264. https://doi.org/10.1590/S0100-40422011000700026
Primel EG, Caldas SS, Marube LC, Escarrone ALV (2017) An overview of advances in dispersive liquid–liquid microextraction for the extraction of pesticides and emerging contaminants from environmental samples. Trends Environ Anal Chem 14:1–18
Rani M, Shanker U, Jassal V (2017) Degradation of hazardous organic dyes in water by nanomaterials. J Environ Manag 190:208–222
Rawtani D, Khatri N, Tyagi S, Pandey G (2018) Nanotechnology-based recent approaches for sensing and remediation of pesticides. J Environ Manag 206:749–762
Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116:1–9, 1
Rocha BA, de Oliveira SR, da Silva RM et al (2019) An eco-friendly sample preparation procedure based on low-density solvent-based air-assisted liquid–liquid microextraction for the simultaneous determination of 21 potential endocrine disruptors in urine samples by liquid chromatography-tandem mass spectrometry. Microchem J 147:207–214
Sajid M, Alhooshani K (2018) Dispersive liquid–liquid microextraction based binary extraction techniques prior to chromatographic analysis: a review. TrAC Trends Anal Chem 108:167–182
Sajid M, Płotka-Wasylka J (2018) Combined extraction and microextraction techniques: Recent trends and future perspectives. Trends Anal Chem 103:74–86. https://doi.org/10.1016/j.trac.2018.03.013
Shoiful A, Ueda Y, Nugroho R, Honda K (2016) Degradation of organochlorine pesticides (OCPs) in water by iron (Fe)-based materials. J Water Process Eng 11:110–117
Suanon F, Tang L, Sheng H, Fu Y, **ang L, Wang Z, Shao X, Mama D, Jiang X, Wang F (2020) Organochlorine pesticides contaminated soil decontamination using TritonX-100-enhanced advanced oxidation under electrokinetic remediation. J Hazard Mater 393:122388. https://doi.org/10.1016/j.jhazmat.2020.122388
Tian H, Li J, Mu Z, Li L, Hao Z (2009) Effect of pH on DDT degradation in aqueous solution using bimetallic Ni/Fe nanoparticles. Sep Purif Technol 66:84–89
Tsai WC, Huang SD (2009) Dispersive liquid–liquid microextraction with little solvent consumption combined with gas chromatography–mass spectrometry for the pretreatment of organochlorine pesticides in aqueous samples. J Chromatogr A 1216:5171–5175
Wacławek S, Silvestri D, Hrabák P, Padil VVT, Torres-Mendieta R, Wacławek M, Černík M, Dionysiou DD (2019) Chemical oxidation and reduction of hexachlorocyclohexanes: a review. Water Res 162:302–319
Wang Z, Peng P, Huang W (2009) Dechlorination of γ-hexachlorocyclohexane by zero-valent metallic iron. J Hazard Mater 166:992–997
Wang Y, Zhang S, Cui W, Meng X, Tang X (2018) Polycyclic aromatic hydrocarbons and organochlorine pesticides in surface water from the Yongding River basin, China: seasonal distribution, source apportionment, and potential risk assessment. Sci Total Environ 618:419–429
Wu Q, Wang C, Liu Z, Wu C, Zeng X, Wen J, Wang Z (2009) Dispersive solid-phase extraction followed by dispersive liquid–liquid microextraction for the determination of some sulfonylurea herbicides in soil by high-performance liquid chromatography. J Chromatogr A 1216:5504–5510
Xu L, Wang J (2012) Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ Sci Technol 46:10145–10153
Xu LJ, Miao XH, Li HP et al (2019) Ultrasound-assisted dispersive liquid–liquid microextraction based on solidification of floating organic droplet for determination of organochlorine compounds in sediment. Microchem J 150:1–9
Yan H, Wang H (2013) Recent development and applications of dispersive liquid–liquid microextraction. J Chromatogr A 1295:1–15
Yang SC, Lei M, Chen TB, Li XY, Liang Q, Ma C (2010) Application of zerovalent iron (Fe0) to enhance degradation of HCHs and DDX in soil from a former organochlorine pesticides manufacturing plant. Chemosphere 79:727–732
Yenisoy-Karakaş S (2006) Validation and uncertainty assessment of rapid extraction and clean-up methods for the determination of 16 organochlorine pesticide residues in vegetables. Anal Chim Acta 571:298–307
Yu B, Zeng J, Gong L, Yang XQ, Zhang L, Chen X (2008) Photocatalytic degradation investigation of dicofol. Chin Sci Bull 53:27–32
Zacharis CK, Tzanavaras PD, Roubos K, Dhima K (2010) Solvent-based de-emulsification dispersive liquid–liquid microextraction combined with gas chromatography–mass spectrometry for determination of trace organochlorine pesticides in environmental water samples. J Chromatogr A 1217:5896–5900
Zhang N, Gao J, Huang C, Liu W, Tong P, Zhang L (2016) In situ hydrothermal growth of ZnO/g-C3N4 nanoflowers coated solid-phase microextraction fibers coupled with GC-MS for determination of pesticides residues. Anal Chim Acta 934:122–131
Zhao Z, Zhang L, Wu J et al (2011) Evaluation of dispersive liquid–liquid microextraction coupled with gas chromatography–microelectron capture detection (GC-μECD) for the determination of organochlorine pesticides in water samples. Anal Sci 27:547
Zuloaga O, Olivares M, Navarro P, Vallejo A, Prieto A (2015) Dispersive liquid–liquid microextraction: trends in the analysis of biological samples. Bioanalysis 7:2211–2225
Acknowledgments
The authors are grateful for the financial support and research fellowships that were received.
Funding
This research was supported by the Foundation for Research of the State of Minas Gerais (FAPEMIG), National Council for Scientific and Technological Development (CNPq), Federal District Research Support Foundation (FAP-DF, 193.000.713/2016), and the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (CAPES).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carvalho, R.R.R., Rodriguez, M.D.V.R., Franco, E.S. et al. DLLME-SFO-GC-MS procedure for the determination of 10 organochlorine pesticides in water and remediation using magnetite nanoparticles. Environ Sci Pollut Res 27, 45336–45348 (2020). https://doi.org/10.1007/s11356-020-10285-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10285-2