Abstract
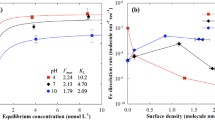
The pesticide gibberellic acid (GA3) is a potential endocrine disruptor and environmental toxin; therefore, research into its environmental fate is warranted. Batch studies were conducted to investigate the sorption and desorption characteristics of GA3 on aquifer media. The results demonstrated special sorption characteristic of GA3 on ferrihydrite compared to goethite, hematite, montmorillonite, and kaolinite, where the sorption kinetics of GA3 on ferrihydrite was fitted well with the pseudo-second-order, Elovich, and intra-particle diffusion models. The sorption kinetics of GA3 on ferrihydrite indicated an initial high sorption rate followed by a slow reaction process. The initial high GA3 sorption rate may be related to electrostatic sorption and surface complexation reactions on the outer surfaces and at the macropore entrances of ferrihydrite. While the slow step was controlled by GA3 diffusion into mesopore of ferrihydrite. Analysis of the desorption hysteresis indicated a high hysteresis index (HI) ranging from 0.68 to 17.32, and a low desorption percentage ranging from 18 to 48%. After sufficient desorption, the calculated maximum residual GA3 quantity due to surface complexation reactions with the ferrihydrite coordinated unsaturated sites was 9.05 ± 0.12 mg g−1. The calculated maximum quantity of GA3 trapped within the mesopore was 16.23 ± 0.91 mg g−1.

Schematic overview of GA3 sorption and desorption on five minerals in groundwater





Similar content being viewed by others
References
Abdellaoui K, Halima-Kamel MB, MHB H (2009) Physiological effects of gibberellic acid on the reproductive potential of Locusta migratoria migratoria. Tunis J Plant Prot 4:67–76
Abramian L, El-Rassy H (2009) Adsorption kinetics and thermodynamics of azo-dye Orange II onto highly porous titania aerogel. Chem Eng J 150:403–410. doi:10.1016/j.cej.2009.01.019
Boga A, Binokay S, Sertdemir Y (2009) The toxicity and teratogenicity of gibberellic acid (GA(3)) based on the frog embryo teratogenesis assay-Xenopus (FETAX). Turk J Biol 33:181–188. doi:10.3906/biy-0807-30
Brennan FP, Moynihan E, Griffiths BS, Hillier S, Owen J, Pendlowski H, Avery LM (2014) Clay mineral type effect on bacterial enteropathogen survival in soil. Sci Total Environ 468:302–305. doi:10.1016/j.scitotenv.2013.08.037
Cadkova E, Komarek M, Kaliszova R, Szakova J, Vanek A, Bordas F, Bollinger J-C (2013) The influence of copper on tebuconazole sorption onto soils, humic substances, and ferrihydrite. Environ Sci Pollut Res 20:4205–4215. doi:10.1007/s11356-012-1198-0
Celik I, Tuluce Y, Isik I (2007a) Evalution of toxicity of abcisic acid and gibberellic acid in rats: 50 days drinking water study. J Enzym Inhib Med Chem 22:219–226. doi:10.1080/14756360600988955
Celik I, Turker M, Tuluce Y (2007b) Abcisic acid and gibberellic acid cause increased lipid peroxidation and fluctuated antioxidant defense systems of various tissues in rats. J Hazard Mater 148:623–629. doi:10.1016/j.jhazmat.2007.03.018
Celis R, Hermosin MC, Cox L, Cornejo J (1999) Sorption of 2,4-dichlorophenoxyacetic acid by model particles simulating naturally occurring soil colloids. Environ Sci Technol 33:1200–1206. doi:10.1021/es980659t
Chung S-G, Ryu J-C, Song M-K, An B, Kim S-B, Lee S-H, Choi J-W (2014) Modified composites based on mesostructured iron oxyhydroxide and synthetic minerals: a potential material for the treatment of various toxic heavy metals and its toxicity. J Hazard Mater 267:161–168. doi:10.1016/j.jhazmat.2013.12.056
Clausen L, Fabricius I (2001) Atrazine, isoproturon, mecoprop, 2,4-D, and bentazone adsorption onto iron oxides. J Environ Qual 30:858–869
Colombo C, Barron V, Torrent J (1994) Phosphate adsorption and desorption in relation to morphology and crystal properties of synthetic hematites. Geochim Cosmochim Acta 58:1261–1269. doi:10.1016/0016-7037(94)90380-8
Ding G, Rice JA (2011) Effect of lipids on sorption/desorption hysteresis in natural organic matter. Chemosphere 84:519–526. doi:10.1016/j.chemosphere.2011.03.009
Dixit S, Hering JG (2003) Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Technol 37:4182–4189. doi:10.1021/es030309t
Elmofty MM, Sakr SA, Rizk AM, Moussa EA (1994) Carcinogenic effect of gibberellin A(3) in Swiss albino mice. Nutr Cancer-an Int J 21:183–190
Gu C, Karthikeyan KG (2005) Sorption of the antimicrobial ciprofloxacin to aluminum and iron hydrous oxides. Environ Sci Technol 39:9166–9173. doi:10.1021/es051190f
Hanna K, Carteret C (2007) Sorption of 1-hydroxy-2-naphthoic acid to goethite, lepidocrocite and ferrihydrite: batch experiments and infrared study. Chemosphere 70:178–186. doi:10.1016/j.chemosphere.2007.06.035
Hermosin MC, Martin P, Cornejo J (1993) Adsorption mechanisms of monobutyltin in clay-minerals. Environ Sci Technol 27:2606–2611. doi:10.1021/es00048a044
Huang WL, Weber WJ (1997) A distributed reactivity model for sorption by soils and sediments. 10. Relationships between desorption, hysteresis, and the chemical characteristics of organic domains. Environ Sci Technol 31:2562–2569. doi:10.1021/es960995e
Huang WL, Yu H, Weber WJ (1998) Hysteresis in the sorption and desorption of hydrophobic organic contaminants by soils and sediments—1. A comparative analysis of experimental protocols. J Contam Hydrol 31:129–148. doi:10.1016/s0169-7722(97)00056-9
Iglesias A, Lopez R, Gondar D, Antelo J, Fiol S, Arce F (2010) Adsorption of MCPA on goethite and humic acid-coated goethite. Chemosphere 78:1403–1408. doi:10.1016/j.chemosphere.2009.12.063
Ijagbemi CO, Baek M-H, Kim D-S (2009) Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J Hazard Mater 166:538–546. doi:10.1016/j.jhazmat.2008.11.085
** Z, Zhong C, Lan G (2005) Gibberellins effect on growth and development of suckling mice. Practical Preventive Medicine 2:003
Jordan N, Ritter A, Scheinost AC, Weiss S, Schild D, Huebner R (2014) Selenium(IV) uptake by maghemite (gamma-Fe2O3). Environ Sci Technol 48:1665–1674. doi:10.1021/es4045852
Kim M, Hyun S (2014) Effect of surface coordination on 2,4-D sorption by kaolinite from methanol/water mixture. Chemosphere 103:329–335. doi:10.1016/j.chemosphere.2013.12.028
Leone P, Gennari M, Negre M, Boero V (2001) Role of ferrihydrite in adsorption of three imidazolinone herbicides. J Agric Food Chem 49:1315–1320. doi:10.1021/jf000913c
Li J, **a J-T, Liu H-B, He J-L, Zhang J-H (2011) Nanoporous carbon synthesized from sol-gel template for adsorbing gibberellic acid in aqueous solution. Mater Sci Eng C-Mater Biol Appl 31:1313–1319. doi:10.1016/j.msec.2011.04.013
Luengo C, Puccia V, Avena M (2011) Arsenate adsorption and desorption kinetics on a Fe(III)-modified montmorillonite. J Hazard Mater 186:1713–1719. doi:10.1016/j.jhazmat.2010.12.074
Mander LN (1992) The chemistry of gibberellins: an overview. Chem Rev 92:573–612. doi:10.1021/cr00012a005
Michel FM et al (2007) The structure of ferrihydrite, a nanocrystalline material. Science 316:1726–1729. doi:10.1126/science.1142525
Peak D, Regier T (2012) Direct observation of tetrahedrally coordinated Fe(III) in ferrihydrite. Environ Sci Technol 46:3163–3168. doi:10.1021/es203816x
Sannino F, Iorio M, Addorisio V, De Martino A, Capasso R (2009) Comparative study on the sorption capacity of cyhalofop acid on polymerin, ferrihydrite, and on a ferrihydrite-polymerin complex. J Agric Food Chem 57:5461–5467. doi:10.1021/jf900573f
Schwertmann HCU, Cornell RM (2000) Iron oxides in the laboratory: preparation and characterization. Wiley-VCH, Weinheim, pp 105
Shi Y (2011) Study on detection of gibberellic A3 by HPLC method and on oxidation damage in mice. Agricultural University of Hebei Province, Hebei Province
de Souza IRP, MacAdam JW (2001) Gibberellic acid and dwarfism effects on the growth dynamics of B73 maize (Zea mays L.) leaf blades: a transient increase in apoplastic peroxidase activity precedes cessation of cell elongation. J Exp Bot 52:1673–1682. doi:10.1093/jexbot/52.361.1673
Tang H, Zhou W, Zhang L (2012) Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater 209:218–225. doi:10.1016/j.jhazmat.2012.01.010
Thakkar M, Randhawa V, Mitra S, Wei L (2015) Synthesis of diatom-FeOx composite for removing trace arsenic to meet drinking water standards. J Colloid Interface Sci 457:169–173. doi:10.1016/j.jcis.2015.07.003
Troudi A, Ben Amara I, Soudani N, Bouaziz H, Boudawara T, Zeghal N (2011) Oxidative stress induced by gibberellic acid in bone of suckling rats. Ecotoxicol Environ Saf 74:643–649. doi:10.1016/j.ecoenv.2010.10.010
Werner D, Garratt JA, Pigott G (2013) Sorption of 2,4-D and other phenoxy herbicides to soil, organic matter, and minerals. J Soils Sediments 13:129–139. doi:10.1007/s11368-012-0589-7
Xu N, Christodoulatos C, Braida W (2006) Modeling the competitive effect of phosphate, sulfate, silicate, and tungstate anions on the adsorption of molybdate onto goethite. Chemosphere 64:1325–1333. doi:10.1016/j.chemosphere.2005.12.043
Yu C, Bi E (2015) Roles of functional groups of naproxen in its sorption to kaolinite. Chemosphere 138:335–339. doi:10.1016/j.chemosphere.2015.06.023
Zeng H, Fisher B, Giammar DE (2008) Individual and competitive adsorption of arsenate and phosphate to a high-surface-area iron oxide-based sorbent. Environ Sci Technol 42:147–152. doi:10.1021/es071553d
Zhang JS, Stanforth R (2005) Slow adsorption reaction between arsenic species and goethite (alpha-FeOOH): diffusion or heterogeneous surface reaction control. Langmuir 21:2895–2901. doi:10.1021/la047636e
Zhao JM, Huggins FE, Feng Z, Huffman GP (1994) Ferrihydrite—surface-structure and its effects on phase-transformation. Clay Clay Miner 42:737–746
Zhao Y, Gu X, Li S, Han R, Wang G (2015) Insights into tetracycline adsorption onto kaolinite and montmorillonite: experiments and modeling. Environ Sci Pollut Res 22:17031–17040. doi:10.1007/s11356-015-4839-2
Zhou C, Wu Q, Lei T, Negulescu JI (2014) Adsorption kinetic and equilibrium studies for methylene blue dye by partially hydrolyzed polyacrylamide/cellulose nanocrystal nanocomposite hydrogels. Chem Eng J 251:17–24. doi:10.1016/j.cej.2014.04.034
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 41302199) and the Elite Scholar Program (Program E) of Tian** University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Guilherme L. Dotto
Highlights
• GA3 sorption on ferrihydrite is the main process for its retardation in vadose zone
• The desorption hysteresis of GA3 on ferrihydrite is divided into three phases
• Surface complexation and mesopore entrapment result in GA3 hysteresis
• GA3 surface complexation is 9.05 ± 0.12 mg/g and pore entrapment is 16.23 ± 0.91 mg/g
Novelty Statement
The pesticide gibberellic acid (GA3) is a potential endocrine disruptor and environmental toxin; therefore, research into its environmental fate in subsurface soil is warranted. This paper investigated the sorption and desorption characteristics of GA3 on five common soil minerals including ferrihydrite, goethite, hematite, montmorillonite, and kaolinite. The results demonstrated that GA3 exhibited remarkable sorption and hysteresis on ferrihydrite but negligible on the other four minerals. These results indicate that GA3 could migrate quickly in subsurface soil and make a higher risk on groundwater safety.
Electronic supplementary material
ESM 1
(DOCX 1533 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Liu, F. & Chen, L. Sorption specificity and desorption hysteresis of gibberellic acid on ferrihydrite compared to goethite, hematite, montmorillonite, and kaolinite. Environ Sci Pollut Res 24, 19068–19075 (2017). https://doi.org/10.1007/s11356-017-9445-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9445-z