Abstract
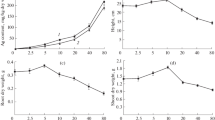
Silver nanoparticles (AgNPs) were extensively used in various fields, particularly in medicine as an antimicrobial agent. The unavoidable and extensive usage of AgNPs in turn accumulates in the environment. Plants are the essential base of ecosystem and are ready to disturb by environmental pollutants. Therefore, in the present study, we have planned to evaluate the impact of biologically synthesized AgNPs on the essential food crop Chinese cabbage (Brassica rapa ssp. pekinensis). The effects of AgNP-induced plant morphological and physiological changes were investigated in different concentrations (100, 250, and 500 mg/L). The results of morphological features showed that AgNPs at lower concentrations (100 mg/L) exhibit growth-stimulating activity, whereas at higher concentrations (250 and 500 mg/L), particularly, 500 mg/L exhibited growth-suppressing activities which are in terms of reduced root, shoot growth, and fresh biomass. The increased reactive oxygen species (ROS) generation, malondialdehyde production, anthocyanin biosynthesis, and decreased chlorophyll content were also more obviously present at higher concentrations of AgNPs. The concentration-dependent DNA damage was observed in the AgNP-treated plants. The molecular responses of AgNPs indicate that most of the genes related to secondary metabolism (glucosinolates, anthocyanin) and antioxidant activities were induced at higher concentrations of AgNP treatment. The dose-dependent phytotoxicity effects of AgNPs were also observed. Taken together, the highest concentration of AgNPs (500 mg/L) could induce growth-suppressing activities via the induction of ROS generation and other molecular changes in B. rapa seedlings.





Similar content being viewed by others
References
Almofti MR, Ichikawa T, Yamashita K, Terada H, Shinohara Y (2003) Silver ion induces a cyclosporine a-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome C. J Biochem 134(1):43–49
Banerjee P, Satapathy M, Mukhopahayay A, Das P (2014) Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresources and Bioprocessing 1:3. http://www.bioresourcesbioprocessing.com/content/1/1/3
Barrena R, Casals E, Colon J, Font X, Sanchez A et al (2009) Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75(7):850–857
Borevitz JO, **a Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12(12):2383–2394
Celenza JL, Quiel JA, Smolen GA, Merrikh H, Silvestro AR, Normanly J, Bender J (2005) The Arabidopsis ATR1 MYB transcription factor controls indolic glucosinolate homeostasis. Plant Physiol 137(1):253–262
Choi O, Hu ZQ (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42(12):4583–4588
El-Temsah YS, Jone EJ (2010) Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol 27(1):42–49
El-Temsah YS, Joner EJ (2012) Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol 27(1):42–49
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37(2):517–531
Faisal M, Saquib Q, Alatar AA, Al-Khedhairy AA, Hegazy AK, Musarrat J (2013) Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J Hazard Mater 250–251:318–332. doi:10.1016/j.jhazmat.2013.01.063
Geisler-Lee J, Wang Q, Yao Y, Zhang W, Geisler M, Li K, Huang Y, Chen Y, Kolmakov A, Ma X (2013) Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 7(3):323–337
Gigolashvili T, Berger B, Flu¨gge UI (2009) Specific and coordinated control of indolic and aliphatic glucosinolate biosynthesis by R2R3-MYB transcription factors in Arabidopsis thaliana. Phytochem Rev 8(1):3–13. doi:10.1007/s11101-008-9112-6
Guo J, Wang MH (2008) Characterization of the phenylalanine ammonia-lyase gene (SlPAL5) from tomato (Solanum lycopersicumL.). Mol Biol Rep 36(6):1579–1585. doi:10.1007/s11033-008-9354-9
Harris AT, Bali (2008) On the formation and extent of uptake of silver nanoparticles by live plants. J Nanoparticle Res 10(4):691–695
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. 1. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–215
Hernandez M, Fernandez-Garcia N, Diaz-Vivancos P, Olmos E (2010) A different role for hydrogen peroxide and the antioxidative system under short and long salt stress in Brassica oleracea roots. J Exp Bot 61(2):521–535
Jahangir M, Kim HK, Choi YH, Verpoorte R (2009) Health-affecting compounds in Brassicaceae. Compr Rev Food Sci Food Saf 8(2):31–43
Jayalakshmi NR, Saraswathi KJT, Vijaya B, Raman DNS, Suresh R (2012) Establishment of enhanced anthocyanin production in Malva sylvestris L. with different induced stress. Int J Pharm Biol Sci 3(4):17–27
Jeong SW, Das PK, Jeoung SC, Song JY, Lee HK, Kim YK, Kim WJ, Park YI, Yoo SD, Choi SB, Choi G, Park YI (2010) Ethylene suppression of sugar-induced anthocyanin pigmentation in Arabidopsis. Plant Physiol 154(3):1514–1531
Kim S, Choi JE, Choi J, Chung KH, Park K, Yi J, Ryu DY (2009) Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol in Vitro 23(6):1076–1084
Kumari M, Mukherjee A, Chandrasekaran N (2009) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407(19):5243–5246
Levard C, Hotze EM, Lowry GV, Brown GE Jr (2012) Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol 46(13):6900–6914
Lin CC, Kao CH (2000) Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul 30(2):151–155
Nair PMG, Park SY, Choi J (2013) Evaluation of the effect of silver nanoparticles and silver ions using stress responsive gene expression in Chironomus riparius. Chemosphere 92(5):592–599
Ma C, Chhikara S, **ng B, Musante C, White JC, Dhankher OP (2013) Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sustain Chem Eng 1(7):768–778
Ma Y, Kuang L, He X, Bai W, Ding Y, Zhang Z, Zhao Y, Chai Z (2010) Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 78(3):273–279
Malecka A, Jarmuszkiewicz W, Tomaszewska B (2001) Antioxidative defense to lead stress in subcellular compartments of pea root cells. Acta Biochim Pol 48(3):687–698
Martínez-Ballesta MC, Moreno DA, Carvajal M (2013) The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int J Mol Sci 14(6):11607–11625. doi:10.3390/ijms140611607
Mikkelsen MD, Naur P, Halkier BA (2004) Arabidopsis mutants in the C–S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J 37(5):770–777
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164(5):601–610
Nair PMG, Chung IM (2014a) Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ Sci Pollut Res 21:8858–8869. doi:10.1016/j.chemosphere.2014.03.056, Epub 2014 May 4
Nair PMG, Chung IM (2014b) Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 112:105–113
Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R et al (2008) Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol 42(23):8959–8964
Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M (1993) Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J 12(9):3679–3684
Padilla G, Cartea ME, Velasco P, de Haro A, Ordás A (2007) Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 68(4):536–545
Piotrowski M, Schemenewitz A, Lopukhina A, Müller A, Janowitz T, Weiler EW et al (2004) Desulfoglucosinolate sulfotransferases from Arabidopsis thaliana catalyze the final step in the biosynthesis of the glucosinolate core structure. J Biol Chem 279(49):50717–50725
Poynton HC, Lazorchak JM, Impellitteri CA, Blalock BJ, Rogers K et al (2012) Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ Sci Technol 46:6288–6296
Qian H, Peng X, Han X, Ren J, Sun L, Fu Z (2013) Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J Environ Sci 25(9):1947–1956
Rico CM, Hong J, Morales MI, Zhao L, Barrios AC, Zhang JY, Peralta-Videa JR, Gardea-Torresdey JL (2013) Effect of cerium oxide nanoparticles on rice: a study involving the antioxidant defense system and in vivo fluorescence imaging. Environ Sci Technol 47(11):5635–5642
Salama HMH (2012) Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int Res J Biotechnol 3(10):190–197
Sharma P, Bhatt D, Zaidi MG, Saradhi PP, Khanna PK et al (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol 167(8):2225–2233
Strader LC, Beisner ER, Bartel B (2009) Silver ions increase auxin efflux independently of effects on ethylene response. Plant Cell 21(11):3585–3590
Wang J, Koo Y, Alexander A, Yang Y, Westerhof S, Zhangm Q, Schnoor JL, Colvin VL, Braam J, Alvarez PJJ (2013) Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ Sci Technol 47(10):5442–5449
Yin L, Colman BP, McGill BM, Wright JP, Bernhardt ES (2012) Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 7(10):e47674. doi:10.1371/journal.pone.0047674
Acknowledgments
This research was supported by Export Promotion Technology Development Program, Ministry of Agriculture, Food and Rural Affairs.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Highlights
• AgNPs acts as growth stimulator at lower concentrations.
• At higher concentrations, AgNPs exhibit growth retardation.
• AgNPs alters genes involved in glucosinolates, anthocyanin, and antioxidants.
• AgNPs showed concentration-dependent phytotoxicity ( ROS, MDA,
ROS, MDA,  chlorophyll contents).
chlorophyll contents).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary figure 1
Characterization of silver nanoparticles using transmission electron microscopy. The size and morphology of silver nanoparticles. (TIFF 705 kb)
Supplementary figure 2
UV–vis absorption spectrum of AgNPs synthesized by Vitex negundo leaf extract taken at 1 to 15 days. (TIFF 77 kb)
Supplementary figure 3
Figure showing the in vivo formation of gradual reactive generation species (ROS) production in the roots of AgNPs treated of Brassica rapa ssp. pekinensis seedlings examined with 3’-(p-hydroxyphenyl) fluorescein staining (4x magnification). The generation of ROS in the roots of B. rapa ssp. pekinensis seedlings treated with different concentrations (from a - d: 0,100, 250 and 500 mg/L) of silver nanoparticles. e - Showing the accumulation of ROS in the root hairs of B. rapa ssp. pekinensis seedlings. Five replicates were analyzed for each treatment. (TIFF 981 kb)
Supplementary figure 4
DNA fragmentation analysis of silver nanoparticles treated and untreated Brassica rapa ssp. pekinensis seedlings; lane 1- 100bp marker DNA (100bp M), lane 2- control (C), lane 3-5 - AgNPs treatment at different concentrations (0, 100, 250 and 500 mg/L) and lane 6- 1 kb marker (M) DNA. (TIFF 582 kb)
Supplementary Table 1
Oligonucleotides used in this study. Gene sequences retrieved from Brassica database (BRAD) and National centre for Biotechnology (NCBI). (DOC 37 kb)
Supplementary Table 2
Metal uptake of Brassica rapa ssp. pekinensis seedlings after 10 days of exposure (μg/L). Values indicate mean ± SD of three replicates per treatment. Significance indicated by P < 0.05. (DOC 27 kb)
Rights and permissions
About this article
Cite this article
Baskar, V., Venkatesh, J. & Park, S.W. Impact of biologically synthesized silver nanoparticles on the growth and physiological responses in Brassica rapa ssp. pekinensis . Environ Sci Pollut Res 22, 17672–17682 (2015). https://doi.org/10.1007/s11356-015-4864-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4864-1