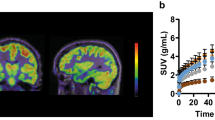
Abstract
Purpose
[18F]UCB-H is a specific positron emission tomography (PET) biomarker for the Synaptic Vesicle protein 2A (SV2A), the binding site of the antiepileptic drug levetiracetam. With a view to optimising acquisition time and simplifying data analysis with this radiotracer, we compared two parameters: the distribution volume (Vt) obtained from Logan graphical analysis using a Population-Based Input Function, and the Standardised Uptake Value (SUV).
Procedures
Twelve Sprague Dawley male rats, pre-treated with three different doses of levetiracetam were employed to develop the methodology. Three additional kainic acid (KA) treated rats (temporal lobe epilepsy model) were also used to test the procedure. Image analyses focused on: (i) length of the dynamic acquisition (90 versus 60 min); (ii) correlations between Vt and SUV over 20-min consecutive time-frames; (iii) and (iv) evaluation of differences between groups using the Vt and the SUV; and (v) preliminary evaluation of the methodology in the KA epilepsy model.
Results
A large correlation between the Vt issued from 60 to 90-min acquisitions was observed. Further analyses highlighted a large correlation (r > 0.8) between the Vt and the SUV. Equivalent differences between groups were detected for both parameters, especially in the 20–40 and 40–60-min time-frames. The same results were also obtained with the epilepsy model.
Conclusions
Our results enable the acquisition setting to be changed from a 90-min dynamic to a 20-min static PET acquisition. According to a better image quality, the 20–40-min time-frame appears optimal. Due to its equivalence to the Vt, the SUV parameter can be considered in order to quantify [18F]UCB-H uptake in the rat brain. This work, therefore, establishes a starting point for the simplification of SV2A in vivo quantification with [18F]UCB-H, and represents a step forward to the clinical application of this PET radiotracer.






Similar content being viewed by others
References
Heurling K, Leuzy A, Jonasson M, Frick A, Zimmer ER, Nordberg A, Lubberink M (2017) Quantitative positron emission tomography in brain research. Brain Res 1670:220–234
Maria Moresco R, Messa C, Lucignani G, Rizzo G G, Todde S, Carla Gilardi M, Grimaldi A, Fazio F (2001) PET in psychopharmacology. Pharmacol Res 44:151–159
Vāvere AL, Scott PJH (2017) Clinical applications of small-molecule PET radiotracers: current progress and future outlook. Semin Nucl Med 47:429–453
Acton PD, Zhuang H, Alavi A (2004) Quantification in PET. Radiol Clin N Am 42:1055–1062 viii
Cunningham VJ, Gunn RN, Matthews JC (2004) Quantification in positron emission tomography for research in pharmacology and drug development. Nucl Med Commun 25:643–646
Fang Y-H, Kao T, Liu R-S, Wu L-C (2004) Estimating the input function non-invasively for FDG-PET quantification with multiple linear regression analysis: simulation and verification with in vivo data. Eur J Nucl Med Mol Imaging 31:692–702
Li F, Joergensen JT, Hansen AE, Kjaer A (2014) Kinetic modeling in PET imaging of hypoxia. Am J Nucl Med Mol Imaging 4:490–506
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, Christman DR (1990) Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 10:740–747
Varga J, Szabo Z (2002) Modified regression model for the Logan plot. J Cereb Blood Flow Metab 22:240–244
Tomasi G, Turkheimer F, Aboagye E (2012) Importance of quantification for the analysis of PET data in oncology: review of current methods and trends for the future. Mol Imaging Biol 14:131–146
Kinahan PE, Fletcher JW (2010) Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR 31:496–505
Bahri MA, Plenevaux A, Aerts J, Bastin C, Becker G, Mercier J, Valade A, Buchanan T, Mestdagh N, Ledoux D, Seret A, Luxen A, Salmon E (2017) Measuring brain synaptic vesicle protein 2A with positron emission tomography and [18F]UCB-H. TRCI 3:481–486
Zanotti-Fregonara P, Chen K, Liow J-S, Fujita M, Innis RB (2011) Image-derived input function for brain PET studies: many challenges and few opportunities. J Cereb Blood Flow Metab 31:1986–1998
Rissanen E, Tuisku J, Luoto P, Arponen E, Johansson J, Oikonen V, Parkkola R, Airas L, Rinne JO (2015) Automated reference region extraction and population-based input function for brain [11C]TMSX PET image analyses. J Cereb Blood Flow Metab 35:157–165
Mabrouk R, Strafella AP, Knezevic D, Ghadery C, Mizrahi R, Gharehgazlou A, Koshimori Y, Houle S, Rusjan P (2017) Feasibility study of TSPO quantification with [18F]FEPPA using population-based input function. PLoS One 12:e0177785
Zanotti-Fregonara P, Hirvonen J, Lyoo CH, Zoghbi SS, Rallis-Frutos D, Huestis MA, Morse C, Pike VW, Innis RB (2013) Population-based input function modeling for [18F]FMPEP-d 2, an inverse agonist radioligand for cannabinoid CB1 receptors: validation in clinical studies. PLoS One 8:e60231
Zanotti-Fregonara P, Hines CS, Zoghbi SS, Liow JS, Zhang Y, Pike VW, Drevets WC, Mallinger AG, Zarate CA Jr, Fujita M, Innis RB (2012) Population-based input function and image-derived input function for [11C](R)-rolipram PET imaging: methodology, validation and application to the study of major depressive disorder. Neuroimage 63:1532–1541
Zanotti-Fregonara P, Maroy R, Peyronneau M-A, Trebossen R, Bottlaender M (2012) Minimally invasive input function for 2-18F-fluoro-A-85380 brain PET studies. Eur J Nucl Med Mol Imaging 39:651–659
Zanderigo F, Ogden RT, Parsey RV (2013) Reference region approaches in PET: a comparative study on multiple radioligands. J Cereb Blood Flow Metab 33:888–897
Lammertsma AA (2017) Forward to the past: the case for quantitative PET imaging. J Nucl Med 58:1019–1024
Lucignani G, Paganelli G, Bombardieri E (2004) The use of standardized uptake values for assessing FDG uptake with PET in oncology: a clinical perspective. Nucl Med Commun 25:651–656
Strauss LG, Dimitrakopoulou-Strauss A, Haberkorn U (2003) Shortened PET data acquisition protocol for the quantification of 18F-FDG kinetics. J Nucl Med 44:1933–1939
Dimitrakopoulou-Strauss A, Pan L, Strauss LG (2012) Quantitative approaches of dynamic FDG-PET and PET/CT studies (dPET/CT) for the evaluation of oncological patients. Cancer Imaging 12:283–289
Lockhart SN, Baker SL, Okamura N, Furukawa K, Ishiki A, Furumoto S, Tashiro M, Yanai K, Arai H, Kudo Y, Harada R, Tomita N, Hiraoka K, Watanuki S, Jagust WJ (2016) Dynamic PET measures of tau accumulation in cognitively normal older adults and Alzheimer’s disease patients measured using [18F] THK-5351. PLoS One 11:e0158460
Lopes Alves I, Vállez García DV, Parente A, et al. (2017) Parametric imaging of [11C]flumazenil binding in the rat brain. Mol Imaging Biol 1–10
Bretin F, Warnock G, Bahri MA, Aerts J, Mestdagh N, Buchanan T, Valade A, Mievis F, Giacomelli F, Lemaire C, Luxen A, Salmon E, Seret A, Plenevaux A (2013) Preclinical radiation dosimetry for the novel SV2A radiotracer [18F]UCB-H. EJNMMI Res 3:35
Warnock GI, Aerts J, Bahri MA, Bretin F, Lemaire C, Giacomelli F, Mievis F, Mestdagh N, Buchanan T, Valade A, Mercier J, Wood M, Gillard M, Seret A, Luxen A, Salmon E, Plenevaux A (2014) Evaluation of 18F-UCB-H as a novel PET tracer for synaptic vesicle protein 2A in the brain. J Nucl Med 55:1336–1341
Bretin F, Bahri MA, Bernard C, Warnock G, Aerts J, Mestdagh N, Buchanan T, Otoul C, Koestler F, Mievis F, Giacomelli F, Degueldre C, Hustinx R, Luxen A, Seret A, Plenevaux A, Salmon E (2015) Biodistribution and radiation dosimetry for the novel SV2A radiotracer [18F]UCB-H: first-in-human study. Mol Imaging Biol 17:557–564
Becker G, Warnier C, Serrano ME, Bahri MA, Mercier J, Lemaire C, Salmon E, Luxen A, Plenevaux A (2017) Pharmacokinetic characterization of [18F]UCB-H PET radiopharmaceutical in the rat brain. Mol Pharm 14:2719–2725
Kaminski RM, Gillard M, Klitgaard H (2012) Targeting SV2A for discovery of antiepileptic drugs. In: Jasper’s Basic Mechanisms of the Epilepsies
Klitgaard H, Verdru P (2007) Levetiracetam: the first SV2A ligand for the treatment of epilepsy. Expert Opin Drug Discovery 2:1537–1545
Nicolas J-M, Hannestad J, Holden D, Kervyn S, Nabulsi N, Tytgat D, Huang Y, Chanteux H, Staelens L, Matagne A, Mathy FX, Mercier J, Stockis A, Carson RE, Klitgaard H (2016) Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia 57:201–209
Mercier J, Provins L, Valade A (2017) Discovery and development of SV2A PET tracers: potential for imaging synaptic density and clinical applications. Drug Discov Today Technol 25:45–52
Rabiner EIA (2017) Imaging synaptic density: a different look at neurological diseases. J Nucl Med
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE (1998) Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res 31:73–84
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Gano LB, Liang L-P, Ryan K, Michel CR, Gomez J, Vassilopoulos A, Reisdorph N, Fritz KS, Patel M (2018) Altered mitochondrial acetylation profiles in a kainic acid model of temporal lobe epilepsy. Free Radic Biol Med 123:116–124
Bertoglio D, Amhaoul H, Van Eetveldt A et al (2017) Kainic acid-induced post-status epilepticus models of temporal lobe epilepsy with diverging seizure phenotype and neuropathology. Front Neurol 8:588
Van Nieuwenhuyse B, Raedt R, Sprengers M et al (2015) The systemic kainic acid rat model of temporal lobe epilepsy: long-term EEG monitoring. Brain Res 1627:1–11
Warnier C, Lemaire C, Becker G, Zaragoza G, Giacomelli F, Aerts J, Otabashi M, Bahri MA, Mercier J, Plenevaux A, Luxen A (2016) Enabling efficient positron emission tomography (PET) imaging of synaptic vesicle glycoprotein 2A (SV2A) with a robust and one-step radiosynthesis of a highly potent 18F-labeled ligand (18[F]UCB-H). J Med Chem 59:8955–8966
Kim JS, Lee JS, Im KC, Kim SJ, Kim SY, Lee DS, Moon DH (2007) Performance measurement of the microPET focus 120 scanner. J Nucl Med 48:1527–1535
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England) 1:307–310
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8:135–160
Ludbrook J (2008) Statistics in biomedical laboratory and clinical science: applications, issues and pitfalls. Med Princ Pract 17:1–13
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd editio. Lawrence Erlbaum Associates
van Vliet EA, Aronica E, Redeker S, Boer K, Gorter JA (2009) Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia 50:422–433
Bajjalieh SM, Peterson K, Linial M, Scheller RH (1993) Brain contains two forms of synaptic vesicle protein 2. Proc Natl Acad Sci U S A 90:2150–2154
Ziai P, Hayeri MR, Salei A, Salavati A, Houshmand S, Alavi A, Teytelboym OM (2016) Role of optimal quantification of FDG PET imaging in the clinical practice of radiology. RadioGraphics 36:481–496
Acknowledgments
We would like to thank Miguel Manuel de Villena for the English revision of the manuscript. The authors are grateful to the Nucleis ULiège spin-off technicians for their help producing [18F]UCB-H.
Funding
This work was funded by University of Liège grant 13/17-07 and UCB BioPharma as partners. MES is supported by ULiege ARC 13/17 07 grant. AP is research director from FRS-FNRS Belgium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Ethical Approval
All animal experiments were performed according to the Helsinki declaration and conducted in accordance with the European guidelines for care of laboratory animals (2010/63/EU). All procedures were reviewed and approved by the Institutional Animal Care and Use Ethics Committee of the University of Liege, Belgium.
Rights and permissions
About this article
Cite this article
Serrano, M.E., Bahri, M.A., Becker, G. et al. Quantification of [18F]UCB-H Binding in the Rat Brain: From Kinetic Modelling to Standardised Uptake Value. Mol Imaging Biol 21, 888–897 (2019). https://doi.org/10.1007/s11307-018-1301-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1301-0