Abstract
Purpose
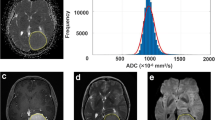
Presurgical grading, estimation of growth kinetics, and other prognostic factors are becoming increasingly important for selecting the best therapeutic approach for meningioma patients. Diffusion-weighted imaging (DWI) provides microstructural information and reflects tumor biology. A novel DWI approach, histogram profiling of apparent diffusion coefficient (ADC) volumes, provides more distinct information than conventional DWI. Therefore, our study investigated whether ADC histogram profiling distinguishes low-grade from high-grade lesions and reflects Ki-67 expression and progesterone receptor status.
Procedures
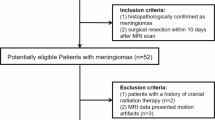
Pretreatment ADC volumes of 37 meningioma patients (28 low-grade, 9 high-grade) were used for histogram profiling. WHO grade, Ki-67 expression, and progesterone receptor status were evaluated. Comparative and correlative statistics investigating the association between histogram profiling and neuropathology were performed.
Results
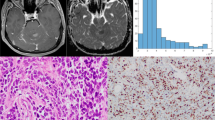
The entire ADC profile (p10, p25, p75, p90, mean, median) was significantly lower in high-grade versus low-grade meningiomas. The lower percentiles, mean, and modus showed significant correlations with Ki-67 expression. Skewness and entropy of the ADC volumes were significantly associated with progesterone receptor status and Ki-67 expression. ROC analysis revealed entropy to be the most accurate parameter distinguishing low-grade from high-grade meningiomas.
Conclusions
ADC histogram profiling provides a distinct set of parameters, which help differentiate low-grade versus high-grade meningiomas. Also, histogram metrics correlate significantly with histological surrogates of the respective proliferative potential. More specifically, entropy revealed to be the most promising imaging biomarker for presurgical grading. Both, entropy and skewness were significantly associated with progesterone receptor status and Ki-67 expression and therefore should be investigated further as predictors for prognostically relevant tumor biological features. Since absolute ADC values vary between MRI scanners of different vendors and field strengths, their use is more limited in the presurgical setting.



Similar content being viewed by others
References
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro-Oncology 18(suppl_5):v1–v75. https://doi.org/10.1093/neuonc/now207
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Böker DK, Meurer H, Gullotta F (1985) Recurring intracranial meningiomas. Evaluation of some factors predisposing for tumor recurrence. J Neurosurg Sci 29:11–17
Marosi C, Hassler M, Roessler K et al (2008) Meningioma. Crit Rev Oncol Hematol 67:153–171
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM (2005) Epidemiology of intracranial meningioma. Neurosurgery 57(6):1088–1095. https://doi.org/10.1227/01.NEU.0000188281.91351.B9
Kshettry VR, Ostrom QT, Kruchko C, al –Mefty O, Barnett GH, Barnholtz–Sloan JS (2015) Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro-Oncology 17(8):1166–1173. https://doi.org/10.1093/neuonc/nov069
Willis J, Smith C, Ironside JW et al (2005) The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol 31:141–149
Pearson BE, Markert JM, Fisher WS, Guthrie BL, Fiveash JB, Palmer CA, Riley K (2008) Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus 24(5):E3. https://doi.org/10.3171/FOC/2008/24/5/E3
van Alkemade H, de Leau M, Dieleman EMT et al (2012) Impaired survival and long-term neurological problems in benign meningioma. Neuro-Oncology 14:658–666
Meixensberger J, Meister T, Janka M et al (1996) Factors influencing morbidity and mortality after cranial meningioma surgery—a multivariate analysis. In: Modern Neurosurgery of Meningiomas and Pituitary Adenomas. Springer Vienna, Vienna, pp 99–101. https://doi.org/10.1007/978-3-7091-9450-8_27
Black P, Kathiresan S, Chung W (1998) Meningioma surgery in the elderly: a case-control study assessing morbidity and mortality. Acta Neurochir 140:1013–1017
Vernooij MW, Ikram MA, Tanghe HL, Vincent AJPE, Hofman A, Krestin GP, Niessen WJ, Breteler MMB, van der Lugt A (2007) Incidental findings on brain MRI in the general population. N Engl J Med 357(18):1821–1828. https://doi.org/10.1056/NEJMoa070972
Steinberger J, Bronheim RS, Vempati P, Oermann EK, Ladner TR, Lee NJ, Kothari P, Caridi JM, Shrivastava RK (2017) Morbidity and mortality of meningioma resection increases in octogenarians. World Neurosurg 109:e16–e23. https://doi.org/10.1016/j.wneu.2017.09.021
Goldbrunner R, Minniti G, Preusser M et al (2016) Review EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 17:e383–e391
Kawahara Y, Nakada M, Hayashi Y et al (2012) Prediction of high-grade meningioma by preoperative MRI assessment. J Neuro-Oncol 108:147–152
Lin B-J, Chou K-N, Kao H-W, Lin C, Tsai WC, Feng SW, Lee MS, Hueng DY (2014) Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg 121(5):1201–1208. https://doi.org/10.3171/2014.7.JNS132359
Yan P-F, Yan L, Hu T-T et al (2017) The potential value of preoperative MRI texture and shape analysis in grading meningiomas: a preliminary investigation. TRANON 10:570–577
Schob S, Frydrychowicz C, Gawlitza M et al (2016) Signal intensities in preoperative MRI do not reflect proliferative activity in meningioma. Transl Oncol 9:274–279
Le Bihan D (2013) Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology 268(2):318–322. https://doi.org/10.1148/radiol.13130420
Schob S, Surov A, Wienke A et al (2016) Correlation between aquaporin 4 expression and different DWI parameters in grade I meningioma. Mol Imaging Biol 19(1):138–142
Nagar VA, Ye JR, Ng WH, Chan YH, Hui F, Lee CK, Lim CCT (2008) Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol 29(6):1147–1152. https://doi.org/10.3174/ajnr.A0996
Surov A, Gottschling S, Mawrin C et al (2015) Diffusion-weighted imaging in meningioma: prediction of tumor grade and association with histopathological parameters. TRANON 8:517–523
Ginat DT, Mangla R, Yeaney G, Wang HZ (2010) Correlation of diffusion and perfusion MRI with Ki-67 in high-grade meningiomas. Am J Roentgenol 195(6):1391–1395. https://doi.org/10.2214/AJR.10.4531
Sanverdi SE, Ozgen B, Oguz KK et al (2012) Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol 81:2389–2395
Schob S, Meyer J, Gawlitza M et al (2016) Diffusion-weighted MRI reflects proliferative activity in primary CNS lymphoma. PLoS One 11(8):e0161386. https://doi.org/10.1371/journal.pone.0161386
Surov A, Caysa H, Wienke A et al (2015) Correlation between different ADC fractions, cell count, Ki-67, total nucleic areas and average nucleic areas in meningothelial meningiomas. Anticancer Res 35:6841–6846
Chen L, Liu M, Bao J et al (2013) The correlation between apparent diffusion coefficient and tumor cellularity in patients: a meta-analysis. PLoS One 8:e79008. https://doi.org/10.1371/journal.pone.0079008.s001
Backer-Grøndahl T, Moen BH, Torp SH (2012) The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol 5(3):231–242
Schob S, Meyer HJ, Pazaitis N, Schramm D, Bremicker K, Exner M, Höhn AK, Garnov N, Surov A (2017) ADC histogram analysis of cervical cancer aids detecting lymphatic metastases—a preliminary study. Mol Imaging Biol 19(6):953–962. https://doi.org/10.1007/s11307-017-1073-y
Rosenkrantz AB (2013) Histogram-based apparent diffusion coefficient analysis: an emerging tool for cervical cancer characterization? Am J Roentgenol 200(2):311–313. https://doi.org/10.2214/AJR.12.9926
Suo S, Zhang K, Cao M et al (2016) Characterization of breast masses as benign or malignant at 3.0T MRI with whole-lesion histogram analysis of the apparent diffusion coefficient. J Magn Reson Imaging 43:894–902
Foroutan P, Kreahling JM, Morse DL, Grove O, Lloyd MC, Reed D, Raghavan M, Altiok S, Martinez GV, Gillies RJ (2013) Diffusion MRI and novel texture analysis in osteosarcoma xenotransplants predicts response to anti-checkpoint therapy. PLoS One 8(12):e82875. https://doi.org/10.1371/journal.pone.0082875
Ryu YJ, Choi SH, Park SJ, Yun TJ, Kim JH, Sohn CH (2014) Glioma: application of whole-tumor texture analysis of diffusion-weighted imaging for the evaluation of tumor heterogeneity. PLoS One 9(9):e108335. https://doi.org/10.1371/journal.pone.0108335
Hsu DW, Efird JT, Hedley-Whyte ET (1997) Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg 86(1):113–120. https://doi.org/10.3171/jns.1997.86.1.0113
Sasaki M, Yamada K, Watanabe Y et al (2008) Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology 249:624–630
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of the medical council of Baden-Württemberg (Ethik-Kommission Landesärztekammer Baden-Württemberg, F-2017-047).
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Information
This study acknowledges funding via the Clinician-Scientist-Program of the medical faculty of the University Hospital Leipzig.
Rights and permissions
About this article
Cite this article
Gihr, G.A., Horvath-Rizea, D., Garnov, N. et al. Diffusion Profiling via a Histogram Approach Distinguishes Low-grade from High-grade Meningiomas, Can Reflect the Respective Proliferative Potential and Progesterone Receptor Status. Mol Imaging Biol 20, 632–640 (2018). https://doi.org/10.1007/s11307-018-1166-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-018-1166-2