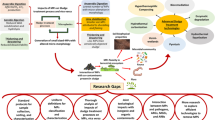
Abstract
Given the society’s continuous reliance on plastic materials, large amounts of micron-sized plastic particles (i.e., microplastics, MPs) reach wastewater treatment plants (WWTPs) every day. Despite their effective removal from influent wastewater, over 90% of MPs in WWTPs are accumulated in sludge. Yet, there is no universally accepted method for quantifying and identifying MPs, obscuring our understanding of this pollution’s extent. Therefore, this study aims to develop a chemical oxygen demand (COD) based repeatable method for MPs analysis in sludge, which is a very complex, MPs-laden by product of WWTPs. The developed method is unique in that it removes the organic substances interfering with polymer analysis by monitoring the COD of sludge. Upon 90% of organic matter removal, MPs are extracted from the medium by a two-step density-based separation, sieved, stained with Nile Red, and counted using fluorescence microscopy. Moreover, quality assurance and quality control strategies including blank preparation and spike-and-recovery test procedures are followed. The protocol ensures a minimum 80% recovery rate of various MPs from both waste activated sludge (WAS) and wastewater samples, aligning with recommended standards. Crucially, the method preserves the chemical identity of MPs. Application of the protocol revealed that urban WWTP WAS had 475 MPs/g TS; industrial influent and effluents wastewater had 73 and 26 MPs/L; and industrial secondary and dewatered sludge had 114 and 132 MPs/g TS, consistent with the literature. This demonstrates the method’s robustness by revealing MPs reduction within the WWTP process and sludge accumulation as treatment progresses.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plastics are one of the most versatile materials of the modern age with an ever-increasing demand for their production (Crawford & Quinn, 2016). The intensive production, extensive use, and mismanagement of plastics have led them to enter the environment for decades. Since the 1950s, approximately 6.9 billion tonnes of plastic materials ended up being waste, and two-thirds of them were either dumped into landfills or released into the environment (Ali et al., 2021). The accumulating plastic debris gradually degrades via natural weathering mechanisms forming highly persistent microplastics (MPs, particle size < 5 mm) (Chamas et al., 2020). MPs pollution threatens the ecosystem health through biological disruption, leaching of toxic chemicals, and sorption and transfer of hazardous substances into biota (Reimonn et al., 2019).
MPs reach wastewater treatment plants (WWTPs) in great amounts from a variety of sources such as households, industrial facilities, agricultural activities, transportation, and landfills. Although WWTPs effectively remove them from the liquid stream, huge amounts of MPs are still released into the environment via effluent due to highly loaded influent wastewater. Additionally, over 90% of MPs in wastewater are transferred to sludge, which yields 510 to 495,000 MPs in each kg dry weight of sludge. Considering the inefficiency of conventional sludge treatment techniques in MPs degradation, 106 to 1014 of particles are released into the environment from a WWTP via disposal and beneficial use of sludge on an annual basis (Hatinoğlu & Sanin, 2021). Multiple orders of magnitude differences between the concentrations reported by different studies can be ascribed to variations in urbanization, population, society’s habits, seasonality, and the treatment units contained at the studied WWTP. Additionally, the lack of consistency in sampling, pretreatment, and analysis methods hampers our ability to compare the results among studies around the world. Therefore, it is imperative to follow a commonly accepted method to reveal the worldwide extent of sludge-related MPs pollution by making a fair comparison of results generated in different laboratories.
Despite the ever-growing concerns about MPs in environmental systems, there is currently no globally accepted and applied standard method for MPs detection. Recently, ASTM has published two standard methods for sampling (ASTM International, 2020a) and preparation (ASTM International, 2020b) of samples to be analyzed for MPs. These methods address drinking water, surface waters, wastewater influent and effluent, and marine waters, whereas sewage sludge is not covered in these documents. On the other hand, sludge has an organic-rich matrix, different than other matrices mentioned above, which poses different challenges in MPs analysis. Typical sludge sample analysis protocols include sampling, pretreatment, extraction, and identification processes. While these techniques are applicable to other sample types, such as soil, it is important to note that the organic-rich matrix of sludge poses unique challenges in terms of pretreatment and organic matter removal (Hurley et al., 2018; Li et al., 2020; Sakali et al., 2021). An optimized pretreatment process is particularly key to remove organic matter as much as possible so that they do not interfere with the polymer identification process in Fourier-transform infrared (FTIR) analyses or Raman spectroscopy while kee** the polymer structure of MPs unaffected. A harsh pretreatment involving strong oxidants has the potential to decrease the mass of certain MPs by up to 60% (Hurley et al., 2018). Moreover, chemical dosage for pretreatment is generally determined based on qualitative measures (i.e., color of the sample during oxidation) that may cause wasting of chemicals with excess use or ineffective removal of organic matter due to insufficient use of oxidant.
This research seeks to address the critical need for a standardized, efficient, and universally applicable method for the detection and quantification of MPs in sludge. As MPs continue to infiltrate our ecosystems and pose a growing threat to human and environmental health, it is imperative to develop a robust approach tailored to the unique challenges posed by sludge matrices. Our research aims to close the existing gap in methodologies for analyzing MPs, with a particular emphasis on the organic-rich composition of sludge. By utilizing COD as an indicator of organic matter oxidation, our study aims to establish a COD-based method that not only improves the accuracy and reproducibility of MPs analysis, but also contributes to a thorough understanding of the global extent of MPs pollution in sludge. To ensure the versatility and dependability of the method, our research encompasses a vast array of MPs of varying types, sizes, and densities, as well as multiple matrices. The developed method was not only tested for sludge samples but also on an organic matrix devoid of any suspended particles as well as wastewater sampled from two different WWTPs. Ultimately, this study goals to provide an optimized, repeatable, and effective tool for addressing the critical issue of MPs contamination in wastewater sludge, thereby advancing our collective commitment to sustainable waste management practices and environmental protection.
2 Materials and Methods
2.1 Materials
2.1.1 Wastewater and Sludge Samples
This study involves the utilization of a variety of wastewater and sludge samples for method development, recovery experiments, and application/validation of the developed method. The wastewater sample used in method development and plastic recovery experiments was obtained from the membrane bioreactor (MBR) WWTP in Ankara at METU campus that is used to treat wastewater from dormitories and faculty residences at a rate of about 204 m3/day (WW). This comparatively small WWTP was primarily selected due to its proximity to our laboratory as well as being solely of domestic origin. Waste activated sludge (WAS) samples were taken from the sludge return line of the secondary clarifier at the Central WWTP of Ankara in Turkey. This is a conventional biological treatment plant designed for a population equivalent of 4 million inhabitants having 765,000 m3/day of capacity. This WAS was used in method development and plastic recovery experiments as well as MP analysis once the method development was completed. Furthermore, to assess the applicability of the developed method, samples were collected from different points of a large WWTP located in Ankara serving an industrial park. These samples included influent (IWWi) and effluent (IWWe) wastewater, secondary sludge (SS), and centrifuge dewatered sludge (DS). All samples were collected in glass jars having caps covered with aluminum foil to eliminate plastic contamination and stored at 4˚C until use. Characteristics of wastewater and sludge samples that were used in method development and recovery studies are given in Table 1. The reported results are the averages of triplicate analysis with their standard deviations.
2.1.2 Model Microplastics
Polyethylene (PE), polyethylene terephthalate (PET), and polyamide (PA) were used in recovery experiments, which are among the most commonly reported polymer types in wastewater and sludge (Yaseen et al., 2022). Besides, these three MPs have distinctly different densities representing low, medium and high density plastics. Three different size ranges were covered when possible, to represent the common MPs size range. PE MPs were readily obtained in powder form from a water tank producer in Ankara. PET and PA type MPs were obtained by cutting water bottles and nylon fishing line using scissors in laboratory, respectively. Before starting the experiments, all the selected MPs’ types were checked by using FTIR spectroscope and spectrums are given in Fig. S5. Then, the particles belonging to different polymer classes were separately passed through a series of sieves to obtain MPs in the appropriate size ranges. The sizes were further confirmed by observing them under microscope. Details about the model MPs produced and used in this study are summarized in Table 2.
2.2 Methods
2.2.1 Method Development for Microplastics Analysis in WAS
Method development involved accomplishing two primary tasks. First, optimization of pretreatment, extraction, and identification and quantification steps (Fig. 1) and second, validation of the protocol for different polymer types and matrices.
Samples brought to the laboratory were analyzed for COD and solids content before pretreatment with Fenton Oxidation. Literature points out that Fenton Oxidation is strong enough to remove organic matter but not as harsh as strong acid or alkali pretreatment that harm MPs. Fenton oxidation is also fast enough to complete the oxidation reaction within practicable times for routine samples which are much shorter compared to wet peroxide oxidation (Hurley et al., 2018). By these evaluations, no other oxidative treatments were tested and Fenton Oxidation was adopted following a dose- and time-optimization study based on COD changes. The detailed mechanism of this reaction is given in Text S1. After adjusting the pH of the samples to 3, three primary parameters were examined: the H2O2 dosage, the molar ratio of H2O2 to Fe(II), and the reaction time (Fig. 1(b)). These investigations were conducted on 300 mL of continuously stirred wastewater or WAS samples in three steps:
-
i.
The molar ratio (MR) was fixed as 10:1 based on literature (San Sebastian Martinez et al., 2003), then the H2O2 dose was tested by adding 1, 2, and 5 times the theoretically required amount needed to oxidize organics in samples based on initially measured COD values of the wastewater or WAS.
-
ii.
The H2O2 was fixed as theoretically required amount by COD and the effective dose of Fe(II) ions was determined by testing different molar ratios as 10:1, 20:1 and 30:1.
-
iii.
The reaction time was varied from 15 to 120 min.
To determine the COD removal efficiency of each technique tested, 30 mL subsamples were taken from the reaction mixture and immediately neutralized to stop the reaction. The subsamples were let to sit for up to 6 h until Fe(III) flocs nicely settled down, as shown in Fig. S1. The COD content was measured in triplicates in supernatant. Lastly, the residual H2O2 in supernatant, that could interfere with the COD measurement, was determined and the COD correction was done as given in Text S2. MPs samples taken from different test conditions were analyzed by FTIR to investigate any potential harm given.
Following the pretreatment, extraction of MPs from wastewater and WAS samples was achieved through density-based separation process followed by the filtration of the samples through a stack of stainless-steel sieves. The best-performing density-based separation operation was determined by examining the recovery rates achieved for model MPs by one step and two-step processes, which include the use of NaCl only, and successive NaCl and ZnCl2, respectively.
-
i.
In the one-step density separation process, NaCl was added into the sample that completed Fenton Oxidation process at a concentration of 5 M making the density of the mixture 1.15 g/cm3. The salty solution, which was stirred for an hour was then transferred to a separatory funnel and allowed to settle overnight. The next day, uppermost portion of the funnel was collected and filtered through a stack of sieves with mesh sizes of 5 mm, 1 mm, 425 µm and 38 µm, respectively.
-
ii.
Two-step procedure, on the other hand, included one more salt addition step and an associated gravity settling period in the process. Following the settling achieved by NaCl, Fe(OH)3 flocs from the bottom of separatory funnel was taken and fed with ZnCl2 to obtain a concentration of 5 M (1.5 g/cm3) and subjected to the same process in another separatory funnel as shown in Fig. S4. Finally, supernatant of the second funnel was also collected and passed through the aforementioned sieves.
In both techniques, the particles trapped on the sieves (except for 5-mm) were separately backwashed onto black Polycarbonate Track Etched (PCTE) membrane filters (1 µm pore size) using vacuum filtration unit. The comparison of recovery rates achieved by both salting operations resulted that two-step process was the best performing option and ensured capturing both low and high-density MPs.
Identification of MPs was based on examination under fluorescent microscope after staining the filters by Nile Red (Greyhound, CAS No: 7385–67-3), which is a hydrophobic fluorescent dye. Nile Red purchased in powder form was first dissolved in acetone to a concentration of 250 mg/L and then diluted to a concentration of 10 mg/L in different solvents (i.e., ultra-pure water, acetone and n-hexane), to find the compatible one with the PCTE solvent-filter combination. Besides, incubation conditions including time and temperature, as well as the dye amount spread onto the filters were optimized. Furthermore, the suitability of various filters was examined such as polycarbonate track etched (PCTE) membrane filters (1 µm pore size), glass fiber (GF/A) filter, cellulose nitrate (CN) filter, qualitative filter paper (Whatman No 1) and mixed cellulose ester (MCE) filter. To determine their effectiveness, the stained filters were inspected under the ZEISS Axio Scope.A1 Fluorescence Microscope using GFP filter after the incubation period. All microscope counting results were corrected by blank analysis.
2.2.2 Recovery Experiment for Spiked MPs and Analysis of Unknown Samples
Comprehensive MPs recovery experiments were carried out to assure the effectiveness and repeatability of the developed protocol. Model MPs listed in Table 2 were spiked into three different matrices. These were potassium hydrogen phthalate (KHP) solution, as an organic matrix without suspended particulates, influent wastewater of a WWTP, and biological sludge (i.e., WAS) from an urban WWTP. Despite being primarily designed for sludge, the proposed method was tested on the aforementioned three matrices with varying degrees of complexity to evaluate the impact of matrix composition on recovery efficiency. WAS is a viscous and rather dense matrix consisting of microorganisms, organic materials, and inorganic particles. Initial COD contents of KHP, wastewater and WAS were set as 937 ± 9, 665 ± 10 and 3368 ± 45 mg/L, respectively during the repeated tests to address typical values for sludge and wastewater. The model MPs were spiked into 400 mL of samples as a mixture containing 25 particles of each size range and type in triplicate beakers. The wastewater volume was set at 400 mL, which is the maximum quantity that can be represented and still be used in the method given the capacities of all experimental instruments. Then, the samples were subjected to the developed MPs analysis procedure. The recovery efficiencies for model MPs were determined in triplicates by counting of the spiked MPs under a fluorescence microscope and subsequent chemical identification using ATR-FTIR spectroscopy. Besides the model MPs, the background MPs in the natural matrices were quantified through the same procedure. Despite having sizes similar to background MPs, model MPs had distinct shapes enabling us to differentiate them from the background ones under fluorescence microscope. Additionally, COD removal efficiencies were also monitored during recovery experiments.
Once the method development was completed with desired recoveries achieved, different wastewater and sludge samples were analyzed with the same methodology to determine the MP concentrations. First, the developed method was applied to quantify unknown MPs in a WAS sample collected from an urban WWTP. Then, wastewater (i.e., IWWi and IWWe) and sludge samples (i.e., SS and DS) collected from an industrial WWTP were tested following the developed protocol. In line with the recovery tests, COD values of the sludge samples were set to approximately 3000 mg/L by dilution, while wastewater samples were used as is before starting the pretreatment process.
2.2.3 Analytical and Instrumental Methods
COD was determined according to USEPA approved digestion method (Jirka & Carter, 1975). Total solids (TS) content of WAS was analyzed according to Standard Method 2540G (APHA et al., 2017). pH was measured by Standard Method 4500 H (APHA et al., 2017). For the collection of spectra used in this study ATR-FTIR (Thermoscientific Nicolet iS10 FTIR Spectrometer) device was used, in the wavenumber range of 400 and 4000 cm−1, and at a spectral resolution of 4 cm−1 with 32 acquisitions. Acquired spectra were analyzed using Thermofisher OMNIC software with its built-in commercial libraries and two user libraries FLOPP and e-FLOPP (De Frond et al., 2021) for better representation of environmental MPs samples.
3 Results and Discussion
3.1 The COD-Based Optimized Fenton Oxidation
First, the H2O2 dose was tested with theoretically required amount determined by COD and the multiples of that (1, 2, and 5 times for wastewater and 1 and 5 times for sludge samples) were tested by fixing the H2O2 to Fe (II) molar ratio (MR) to 10. Figure 2 shows that, while the COD removal efficiency increases when five times the theoretically required H2O2 dose was used for wastewater samples, it was not performing all that well for the sludge samples. Besides, the theoretical value was found sufficient for both wastewater and sludge. For this reason and to prevent the excessive chemical use, the theoretically required amount of H2O2 dose based on COD analysis was deemed to be satisfactory.
In Fig. 3, the removal efficiencies for the Fenton process in WAS are shown along with the change in COD at various MRs of H2O2 to Fe (II). The H2O2 dosage in this experiment was set as the theoretically required level (by COD). At each MR, COD is found to decline over time as would be predicted; with the first 15 min showing the greatest decrease (Fig. 3(b)). Then, a significantly slower rate of reduction is maintained. Higher molar ratios mean that the amount of Fe(II) used for the reaction gets lower. Fe(II) acts as a catalyst during the peroxide oxidation and speeds up the reaction. Results also indicate that the most efficient molar ratio is MR 10, which removes 90% of COD in 120 min. As seen in Fig. 3(b), MR 10 also offers the best COD elimination efficiency in the quickest amount of time. Because MPs are subjected to chemicals and oxidative conditions during pretreatment, it is crucial to provide the shortest amount of time that results in high COD elimination. Additionally, Fig. 3 demonstrates that for all the MRs used, the majority of organic substances are degraded in 15 min. Despite the fact that the oxidation at MR 30 was generally slower because of the lower amount of Fe(II), performance nearly caught up at the conclusion of the two-hour timeframe. Even if higher removals can be seen at later times (90 or 120 min), the additional reaction time is deemed superfluous and prohibitive. So, 30 min was determined to be the ideal pretreatment period.
3.2 The Optimized Extraction and Identification Procedures
The two methods tested, i.e., single step salt extraction with NaCl only and double step salt extraction with NaCl and ZnCl2 together, yielded different extraction efficiencies. Figure 5 illustrates how the use of a two-step density-based separation exceeded the use of a single salt solution to capture MPs. There were two basic explanations given for this. First, after settling following the Fenton process, some MPs are entrapped in Fe(OH)3 flocs, which results in an underestimation of the MPs concentration in the sample if only one step extraction is used. When ZnCl2 is mixed with the settling flocs, the trapped particles become liberated and begin to float upward. As a result, the sample's floating fraction more accurately matched the MPs' content for following analyses. Furthermore, although being among the most commonly reported polymer types in sludge, MPs with high densities including PVC (polyvinyl chloride), PET, PMMA (Poly(methyl methacrylate)), PU (polyurethane), and PES (polyester) could not float in low density environment achieved by the addition of NaCl. However, all these polymers are captured in the sludge sample when ZnCl2 is added. Literature also recommends sequential extraction in NaI and ZnCl2 to separate both the low and high-density MPs and accurately determine their number despite the high price and relatively toxic properties of these two salts (Okoffo et al., 2019; Silva et al., 2018).
The optimization studies for the staining protocol resulted that n-hexane is the best one among the three tested carrier solvents due to its good performance in staining MPs without posing any damage to the PCTE filter. The PCTE filter was substantially damaged by acetone, and the dye precipitation was observed (Fig. S2). However, due to Nile Red's poor solubility in n-hexane, the stock solution had to be prepared in acetone (Shim et al., 2017). Therefore, a 250 mg/L Nile Red stock solution was prepared in acetone and subsequently diluted to 10 mg/L in n-hexane. Furthermore, 200 µL of the dye was applied to a black PCTE filter and incubated at room temperature for 15 min in the dark to produce the brightest MPs fluorescence.
Even though black PCTE provided good contrast facilitating MPs counting, its low-friction flat surface prevents large particles from being captured on the filter surface. This was especially critical for samples to be transferred to another laboratory for further analysis such as FTIR. To ensure capturing all the particles on the surface, filters (i.e., Glass Fiber Filter, GF/A; Cellulose Nitrate, CN; qualitative filter paper, Whatman No 1; and Mixed Cellulose Ester, MCE) with higher surface roughness were tested. Testing for compatibility with Nile Red resulted that all filters except for MCE retained the dye on their surfaces, which caused high background fluorescence hampering visualization of MPs under Fluorescence Microscope (Fig. S3). So, MCE filter was integrated into the developed analysis method, which also surpasses the PCTE filter with its affordable price.
The optimized and selected parameters of the developed procedure are shown in Fig. 1(b) by green check marks, to show the typical analysis carried out for wastewater and sludge samples. If a wastewater sample was analyzed, then a 400 mL of wastewater sample was taken and used as is without any dilution or adjustment of COD. If a sludge sample was analyzed, the sample was diluted to a COD value of around 3000 mg/L. For the validation step conducted with the use of organic matrix without suspended particles, a KHP solution with a COD of about 1000 mg/L was prepared that represents a COD value that rests in between the COD value of other two matrices. A 0.1 M Fe(II) solution prepared in H2SO4 was added into the samples and the pH of the mixtures were adjusted to 3 using 5 M NaOH solution. The reaction was started by adding theoretically required amount of 35% H2O2 solution, yielding [H2O2]/[Fe(II)] = 10. The solution was mixed at 500 rpm at room temperature for 30 min. The reaction was terminated by neutralizing the pH using 5 M NaOH solution. The samples were subjected to two-step density-based separation process, sieved on different mesh sized stainless steel sieves, and collected on filter papers. Particles kept on filters were stained with 200 µL of Nile Red solution (10 mg/L) and incubated for 15 min in dark. Following incubation, filters were inspected under Fluorescence Microscope for counting and classification of shiny particles based on their size and shape. Quantification results were then corrected by a blank analysis using distilled water.
3.3 Results of Recovery Experiments
To check the effectiveness of the optimized method, known plastics at known concentrations were added into KHP (a simple organic matrix), wastewater and then to sludge (i.e., WAS which contains high suspended solids and high organic matter content). The model MPs extracted during recovery experiment are shown in Fig. 4. The recovery efficiencies achieved in each scenario are given in Fig. 5.
Recovery efficiencies obtained for different polymer types and sizes in (a) KHP, (b) wastewater, and (c) WAS samples. For each polymer type, three columns from the left to the right on the y-axis represents different particle size ranges, which are 106–250 µm, 250–500 µm, and 500–1000 µm, respectively. Calculated standard deviation values for triplicate experiments are shown in Tables S1, S2, and S3
3.3.1 KHP as the Sample Matrix
The method was intended for sludge but additional tests were conducted in variety of organic matrices in differing complexities. The simplest matrix having organic matter but no suspended solids was KHP. Its initial COD was 937 ± 9 mg/L, which was well-fitted to a COD value in between that of the other two matrices. Applying our optimized Fenton Oxidation process provided 74.5% COD removal efficiency. Table S1 and Fig. 5(a) shows that the best MPs recovery results were obtained with the lowest density plastic (i.e., PE). It is seen that the effectiveness of the salts used to extract MPs were highly polymer dependent. For example, NaCl was satisfactory regardless of the size of PE MPs studied. As the density of plastics increased, then the performance of extraction and recovery by NaCl from KHP decreased. Although NaCl alone had been the major contributor at PE; both salts acted as effective extraction solutions. As the size of the PA particles increased, the recovery rate by NaCl increased and that of ZnCl2 decreased. Overall recovery ability of both salts was acceptable, but the higher particle size improved the recovery of PA. At the highest density plastic (i.e., PET), ZnCl2 became the major contributor to overall recovery and NaCl had the minimum contribution. For PET in KHP solution, the individual performance of NaCl was poor and adding ZnCl2 significantly improved the capture rate.
Overall, regardless of the polymer types added into the KHP solution, there was a general declining trend in performance with decreasing particle size. Among all the plastics used, PET with the highest density had the lowest recovery in the smallest size. Relatively lower recovery efficiency obtained in KHP can be attributed to the absence of particulate matter which is the center of nuclei for the growth of Fe(OH)3. It is evident that the presence of particles capturing the plastics before Fenton oxidation stage might be hel** to improve the overall recovery. So, the absence of particulate matter in KHP made the sinking of higher density plastics like PET possible, and this made the salt solution flotation more difficult.
Still, recovery rates were at similar levels with Cashman et al. (2020) who typically obtained < 70% recoveries for most plastics spiked into marine sediments tested with a number of different methods. However, general recoveries obtained in KHP were lower than that is recommended by ASTM (ASTM International, 2020b) which is suggested to be > 80%.
3.3.2 Wastewater as the Sample Matrix
The initial COD of wastewater was 665 ± 10 mg/L and 72% COD removal efficiency was achieved by Fenton Oxidation. Similar to the case of KHP, the performance of NaCl was the best in recovery of PE MPs at the studied particle sizes (Table S2). Also, the integration of ZnCl2 was only needed for the smaller particle size of PE (i.e., 250 – 500 µm); altogether which made the effective and total recovery possible. For PA MPs, slightly different than KHP, significant performance was observed with NaCl alone especially at medium and high particle sizes, showing the importance of the extraction medium. For high particle size of PA, NaCl seemed to be enough; addition of ZnCl2 only mattered and increased recovery for the smallest particle size. The performance of NaCl was again poor for PET MPs, except for the smallest particle size. Oppositely, ZnCl2 addition contributed more to the recovery of larger particle sizes.
Overall, in wastewater, the density and size of plastics did not play such a significant role as in the case of KHP; and the general recoveries were in the range of 85–95% for all plastics. This can be due to the effective Fe(OH)3 flocculation and settling resulting from the presence of particulate matter in wastewater which forms condensation nuclei.
3.3.3 WAS as the Sample Matrix
The initial COD of the WAS sample was 3368 ± 45 mg/L and 97% COD removal efficiency was achieved by Fenton Oxidation. As in line with the previous matrices, the performance of NaCl was satisfactory for the recovery of PE MPs at the studied particle sizes (Table S3). The performance of ZnCl2 was minimal and only helped at the marginal level with the larger particle size of PE this time, different than wastewater. For PA MPs having the two higher sizes significant performance with only NaCl addition (close to 80%) was obtained. For these higher particle sizes, ZnCl2 did not bring any improvement for recovery. Addition of ZnCl2 only mattered and increased the recovery for the smallest particle size of PA, similar to the result obtained for wastewater. In sludge, the performance of NaCl for PA was better than that in wastewater. This may be due to the high concentration of particulate matter – making the medium density inherently suitable for recovery. Generally, ZnCl2 added acted as the smaller contributor to the overall recovery of PA MPs and was more effective in smaller sizes. As in line with the other matrices worked with, ZnCl2 was the major contributor for the recovery of PET, due to its high density. The importance of ZnCl2 even increases as the size of PET increases as the particles become heavier.
In general, recovery efficiencies in sludge were comparable to KHP and wastewater. One can say that as the COD is removed highly, the analysis and visualization of MPs become easier and slightly higher recoveries are obtained. Even though at 80% or slightly lower, the lowest recoveries were experienced with the medium density plastic, PA. PA with density of 1.13–1.15 g/cm3, has the closest density to that of sludge among the three plastics studied. So, the recoveries were lower due to possible separation problems. Similar to wastewater, presence of particulate matter in sludge possibly formed condensation nuclei and helped with further flocculation and recovery. Different than the previous two matrices, the overall recoveries did not seem to be related to particle size, mostly at higher/much higher level than 80%. The theoretical amount of peroxide addition seems to be sufficient for effective recoveries, possibly due to the particulate matter in sludge may form a center of nuclei for the produced flocs to grow, affected the overall capture, and hence the plastic recovery. Furthermore, applying the analysis protocol developed resulted in around 97% of COD removal efficiency. All these findings were considered appropriate in light of the literature. As a result, using this technique yields accurate results for both a particle free sample (i.e., KHP) and more complex matrices (i.e., wastewater and WAS). Two prominent techniques that were incorporated into the strategy are primarily responsible for this accomplishment. First, the improved Fenton Oxidation offers such powerful organic removal efficiency (i.e., COD elimination) that the matrix impact in MPs analysis is eliminated. Second, the two-step density-based separation procedure greatly increases the extraction efficiency of MPs (particularly those with high densities), as the sample is mixed twice on consecutive days, allowing MPs trapped in Fenton Oxidation residuals to float upward.
3.3.4 Changes in FTIR Spectra of the Recovered Microplastics
To observe any possible differences in the polymer chemistry, FTIR spectra were collected at all conditions for all plastics. Differences in IR spectra of PET MPs extracted from different mediums can be seen in Fig. 6 as example spectra. Spectra of all conditions are given in Fig. S5. Overall, some small changes are seen (such as carbon dioxide peaks at 2350 cm−1 possibly originating from the measurement environment, etc.), however these changes did not cause any problems in identification of target MPs in FTIR, and all showed plastics were not affected from the recovery process.
3.4 Quantification of Microplastics in Unknown Samples Using the Developed Method
Once the method development was completed with desired recoveries achieved, different wastewater and sludge samples were analyzed with the same methodology to determine the MP amounts. First, the developed method was applied to quantify unknown MPs in a WAS sample collected from an urban WWTP. The suspected MPs were classified by number as fragment (59.9%), fiber (23.1%), and film (16.9%), which complies with literature findings (Fig. 7(a)). These particles were also grouped into different size classes by number: 38–106 µm (34.6%), 106–250 µm (32.6%), 250-500 µm (31.4%), 500–1000 μm (0.8%), and 1000–5000 μm (0.5%) (Fig. 7(b)). Smaller MPs in sludge dominated over larger ones (500–1000 μm), which can be attributed to that smaller ones are removed from wastewater at a higher rate by adsorption onto settling sludge in sedimentation tank (Liu et al., 2019; Magni et al., 2019; Mason et al., 2016; Talvitie et al., 2017).
Fluorescent particles of size > 500 µm were subjected to FTIR analysis and their polymer types are given in Fig. 7(c). The concentration of MPs in WAS determined was 887 MPs/g TS following blank correction and 475 MPs/g TS following FTIR correction (53.6% are plastics). The measured amount is within the reported range, 510—495,000 MPs/kg (Hatinoğlu & Sanin, 2021). The rather high concentration can be attributed to the source of wastewater, which originated from a metropolitan city with some commercial and industrial wastewater contributions to the plant. Results obtained in this part highlight the importance of reporting MPs in sludge or similar solid matrices. To be able to report the results transparently, expressing concentrations in sludge as mass-based values, rather than volume-based values is necessary. This ensures objectivity and make the results comparable between studies.
Following the measurements of WAS sample obtained from the urban WWTP, samples from a WWTP serving an industrial park were analyzed (Fig. 7(d), (e), (f)). All the industrial WWTP samples showed similar results with the previous findings of WAS about the smallest particles dominating over the larger ones (99% of MPs were between 38–425 µm for both wastewaters, 89% for SS and 93% for DS). IWWi and IWWe showed MPs concentrations of 73 MPs/L and 26 MPs/L following FTIR corrections (average 55% are plastics), respectively. MP concentrations of SS and DS were determined as 114 MPs/g TS and 132 MPs/g TS, respectively, after FTIR corrections (average 55% are plastics). The values were found to be in the range of literature reported for industrial samples (Phuprasert et al., 2021; Saiwaree & Kanokkantapong, 2021). Overall, it can be seen that the quantity of MPs decreases in WW within the WWTP as expected and accumulates in sludge as the process continues.
With these results it is seen that the methodology developed has some strengths, such as yielding acceptable recoveries in different matrices. The method also introduces an automation concept in the pretreatment stage using COD based organic matter and its oxidation-based removal. On the other hand, there is still need for improvement and some future studies to eliminate human intervention in the identification stage of the protocol. The developed method, similar to all MP sampling and measurement methods, is tedious and time-consuming, so some automation of these methods is critically needed. An automation system can be introduced at the sampling point, by adding a flow measuring device and a series of size separation sieves at the dedicated sampling ports. Additionally, automated reaction vessels or robotized flow reactor designs can be introduced to the method. The density separation is the most time-consuming part of this methodology, but it does not necessitate constant supervision and does not require prior laboratory knowledge which may in turn be used to justify not automating this process. Microscopic and FT-IR measurements and their automized versions have been used for decades now that there are robust systems being produced today. While automated microscopes have been used in the biological studies such as referenced in Huang and Murphy (2004), its specific applications in the area of microplastics are limited in the literature and more studies are being published without the use of microscopes but automated µFT-IR systems as used in Primpke et al. (2017), Renner et al. (2020) and Tagg et al. (2015). While these systems do work and are automated, they also mention the analysis and data acquisition times are too long in some cases. This indicates that the microscopy step can be used to decrease the workload of automated µFT-IR systems by prescreening the non-microplastic particles from the filter mediums.
4 Conclusion
A validated method was developed for the analysis of MPs in sludge, based on COD removal as the indicator of organic matter removal. As COD removal increases, the analysis and visualization of MPs become easier, resulting in higher recoveries. The critical findings of the study are listed below:
-
Fenton Oxidation applied at a theoretical amount of H2O2 dosage and MR:10 for 30 min resulted in 97% COD removal in WAS and the two-step density-based separation process captured MPs with densities up to 1.5 g/cm3.
-
The developed method ensured recovery of PE, PA, and PET MPs in sizes of 106 to 5000 µm from WAS at a rate of 94, 80, and 92%, respectively, meeting ASTM’s 80% recovery efficiency recommendation. Additionally, the applied protocol did not compromise our ability to chemically identify studied polymers using FTIR.
-
Following the developed protocol, the concentration of unknown MPs in a WAS sample was determined as 475 MPs/g TS upon the correction of the data by performing a laboratory blank test and FTIR identification.
-
The method was also used in wastewater and sludge samples obtained from an industrial WWTP, yielding concentrations within the range reported in the literature for industrial plants.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ali, S. S., Elsamahy, T., Koutra, E., Kornaros, M., El-Sheekh, M., Abdelkarim, E. A., Zhu, D., & Sun, J. (2021). Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. In Science of the total environment (Vol. 771). Elsevier B.V. https://doi.org/10.1016/j.scitotenv.2020.144719
APHA, AWWA, & WEF. (2017). Standard methods for the examination of water and wastewater (23rd ed.). American Public Health Association, American Water Works Association, Water Environment Federation.
ASTM International. (2020a). D8332–20 standard practice for collection of water samples with high, medium, or low suspended solids for identification and quantification of microplastic particles and fibers. https://doi.org/10.1520/D8332-20
ASTM International. (2020b). D8333–20 standard practice for preparation of water samples with high, medium, or low suspended solids for identification and quantification of microplastic particles and fibers using Raman spectroscopy, IR spectroscopy, or pyrolysis-GC/MS. https://doi.org/10.1520/D8333-20
Cashman, M. A., Ho, K. T., Boving, T. B., Russo, S., Robinson, S., & Burgess, R. M. (2020). Comparison of microplastic isolation and extraction procedures from marine sediments. Marine Pollution Bulletin, 159(July), 111507. https://doi.org/10.1016/j.marpolbul.2020.111507
Chamas, A., Moon, H., Zheng, J., Qiu, Y., Tabassum, T., Jang, J. H., Abu-Omar, M., Scott, S. L., & Suh, S. (2020). Degradation rates of plastics in the environment. ACS Sustainable Chemistry and Engineering, 8(9), 3494–3511. https://doi.org/10.1021/acssuschemeng.9b06635
Crawford, C. B., & Quinn, B. (2016). Microplastic pollutants. In Microplastic pollutants. https://doi.org/10.1016/c2015-0-04315-5
De Frond, H., Rubinovitz, R., & Rochman, C. M. (2021). μATR-FTIR spectral libraries of plastic particles (FLOPP and FLOPP-e) for the analysis of microplastics. Analytical Chemistry, 93(48), 15878–15885. https://doi.org/10.1021/acs.analchem.1c02549
Hatinoğlu, M. D., & Sanin, F. D. (2021). Sewage sludge as a source of microplastics in the environment: A review of occurrence and fate during sludge treatment. Journal of Environmental Management, 295, 113028. https://doi.org/10.1016/j.jenvman.2021.113028
Huang, K., & Murphy, R. F. (2004). From quantitative microscopy to automated image understanding. Journal of Biomedical Optics, 9(5), 893. https://doi.org/10.1117/1.1779233
Hurley, R. R., Lusher, A. L., Olsen, M., & Nizzetto, L. (2018). Validation of a method for extracting microplastics from complex, organic-rich. Environmental Matrices. Environmental Science and Technology, 52(13), 7409–7417. https://doi.org/10.1021/acs.est.8b01517
Jirka, A.M., & Carter, M.J. (1975). Micro semi-automated analysis of surface and wastewaters for chemical oxygen demand. Analytical chemistry, 47(8), 1397–1402. https://doi.org/10.1021/ac60358a004
Li, X., Chen, L., Ji, Y., Li, M., Dong, B., Qian, G., Zhou, J., & Dai, X. (2020). Effects of chemical pretreatments on microplastic extraction in sewage sludge and their physicochemical characteristics. Water Research, 171. https://doi.org/10.1016/j.watres.2019.115379
Liu, X., Yuan, W., Di, M., Li, Z., & Wang, J. (2019). Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chemical Engineering Journal, 362(January), 176–182. https://doi.org/10.1016/j.cej.2019.01.033
Magni, S., Binelli, A., Pittura, L., Avio, C. G., Della Torre, C., Parenti, C. C., Gorbi, S., & Regoli, F. (2019). The fate of microplastics in an Italian Wastewater Treatment Plant. Science of the Total Environment, 652, 602–610. https://doi.org/10.1016/j.scitotenv.2018.10.269
Mason, S. A., Garneau, D., Sutton, R., Chu, Y., Ehmann, K., Barnes, J., Fink, P., Papazissimos, D., & Rogers, D. L. (2016). Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environmental Pollution, 218, 1045–1054. https://doi.org/10.1016/j.envpol.2016.08.056
Okoffo, E. D., O’Brien, S., O’Brien, J. W., Tscharke, B. J., & Thomas, K. V. (2019). Wastewater treatment plants as a source of plastics in the environment: A review of occurrence, methods for identification, quantification and fate. Environmental Science: Water Research and Technology, 5(11), 1908–1931. https://doi.org/10.1039/c9ew00428a
Phuprasert, T., Kanokkantapong, V., & Srithongouthai, S. (2021). The abundance and characteristics of microplastics in central industrial wastewater treatment plants. 26th Int’l Conference on “Biological, Environmental, Medical & Veterinary Sciences” (PCBEM-21-Pattaya), 12–18. https://doi.org/10.17758/URUAE14.AE05212006
Primpke, S., Lorenz, C., Rascher-Friesenhausen, R., & Gerdts, G. (2017). An automated approach for microplastics analysis using focal plane array (FPA) FTIR microscopy and image analysis. Analytical Methods, 9(9), 1499–1511. https://doi.org/10.1039/C6AY02476A
Reimonn, G., Lu, T., Gandhi, N., & Chen, W. T. (2019). Review of microplastic pollution in the environment and emerging recycling solutions. Journal of Renewable Materials, 7(12), 1251–1268. https://doi.org/10.32604/jrm.2019.08055
Renner, G., Schmidt, T. C., & Schram, J. (2020). Automated rapid & intelligent microplastics map** by FTIR microscopy: A python–based workflow. MethodsX, 7, 100742. https://doi.org/10.1016/j.mex.2019.11.015
Saiwaree, S., & Kanokkantapong, V. (2021). Microplastics in industrial wastewater treatment plants: Dynamic distribution, seasonal variation, and removal efficiencies (pp. 103–113). https://doi.org/10.1007/978-3-030-75278-1_10
Sakali, A., Coello, D., Haïlaf, A., Egea-Corbacho, A., Albendín, G., Arellano, J., Brigui, J., Quiroga, J. M., & Rodríguez-Barroso, R. (2021). A new protocol to assess the microplastics in sewage sludge. Journal of Water Process Engineering, 44. https://doi.org/10.1016/j.jwpe.2021.102344
San Sebastian Martinez, N. S., Figuls Fernandez, J., Segura, X. F., & Ferrer, A. S. (2003). Pre-oxidation of an extremely polluted industrial wastewater by the Fenton’s reagent. 101, 315–322. https://doi.org/10.1016/S0304-3894(03)00207-3
Shim, W. J., Hong, S. H., & Eo, S. E. (2017). Identification methods in microplastic analysis: A review. Analytical Methods, 9(9), 1384–1391. https://doi.org/10.1039/c6ay02558g
Silva, A. B., Bastos, A. S., Justino, C. I. L., da Costa, J. P., Duarte, A. C., & Rocha-Santos, T. A. P. (2018). Microplastics in the environment: Challenges in analytical chemistry - A review. Analytica Chimica Acta, 1017, 1–19. https://doi.org/10.1016/j.aca.2018.02.043
Tagg, A. S., Sapp, M., Harrison, J. P., & Ojeda, J. J. (2015). Identification and quantification of microplastics in wastewater using focal plane array-based reflectance micro-FT-IR imaging. Analytical Chemistry, 87(12), 6032–6040. https://doi.org/10.1021/acs.analchem.5b00495
Talvitie, J., Mikola, A., Koistinen, A., & Setälä, O. (2017). Solutions to microplastic pollution – Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Research, 123, 401–407. https://doi.org/10.1016/j.watres.2017.07.005
Yaseen, A., Assad, I., Sofi, M. S., Hashmi, M. Z., & Bhat, S. U. (2022). A global review of microplastics in wastewater treatment plants: Understanding their occurrence, fate and impact. Environmental Research, 212. https://doi.org/10.1016/j.envres.2022.113258
Acknowledgements
This work was supported by Middle East Technical University Scientific Research Projects Grant No: GAP-311-2021-10613.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work was supported by Middle East Technical University Scientific Research Projects Grant No: GAP-311–2021-10613.
Author information
Authors and Affiliations
Contributions
Dilara Hatinoglu: Investigation; Data curation; Lab analysis; Validation; Visualization; Writing – original draft. Irem Simsek: Data curation; Lab analysis; Validation; Visualization; Writing, Oguzhan Altuntas: Data curation; Lab analysis, Ozan Karakurt: Data curation; Visualization; Writing, F. Dilek Sanin: Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hatinoglu, D., Simsek, I., Altuntas, O. et al. Enhancing Microplastics Recovery from Complex Sludge Samples Using COD-Guided Pretreatment. Water Air Soil Pollut 235, 307 (2024). https://doi.org/10.1007/s11270-024-07102-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07102-8