Abstract
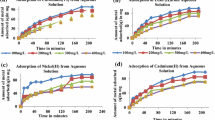
Sorption studies were carried out in a batch system using Cr(III) solution on non-treated and acid-treated white-colored calcium-bentonite (WCa-B). WCa-B, which has the highest cation exchange capacity (119 meq/100 g), was treated with 2 different acids (H2SO4 and HCl) at various concentrations. The surface area was increased approximately 5 times (493 m2/g) without causing deterioration in the crystal structure of bentonite with 1.0 M H2SO4 activation. To determine the chemical and mineralogical composition of the adsorbent, which was characterized with XRD, XRF, Surface Area and Porosity, TG/DTA, FT-IR, SEM, and EDX. For Cr(III) uptake experiments, a batch method was applied. The highest Cr(III) monolayer adsorption capacity was specified as 219.8 mg/g. To Cr(III) adsorption, ΔH°, ΔS°, and ΔG° were designated onto adsorbent and it was found that adsorption is coherent with Langmuir monolayer isotherm, adsorption has an endothermic character, and has spontaneously occurred. By using pseudo-second-order and Lagergren kinetic models, Cr(III) adsorptions kinetic experiments were determined, it was designated that the adsorption was exhibited to be proper for the pseudo-second-order kinetic model.






Similar content being viewed by others
Data Availability
The data sets used and analyzed during the current study is available from the corresponding author on reasonable request.
References
Abbou, B., Lebkiri, I., Ouaddari, H., Elkhattabi, O., Habsaoui, A., Lebkiri, A., & Rifi, E. H. (2021). Kinetic and Thermodynamic Study on Adsorption of Cadmium from Aqueous Solutions Using Natural Clay. Journal of the Turkish Chemical Society Section A: Chemistry, 8(2), 677–692. https://doi.org/10.18596/jotcsa.882016
Adeyemo, A. A., Adeoye, I. O., & Bello, O. S. (2017). Adsorption of dyes using different types of clay: A review. Applied Water Science, 7, 543–568. https://doi.org/10.1007/s13201-015-0322y
Ahmadi, A., Rauf Foroutan, R., Hossein Esmaeili, H., & Tamjidi, S. (2020). The role of bentonite clay and bentonite clay @ MnFe2O4 composite and their physico-chemical properties on the removal of Cr(III) and Cr(VI) from aqueous media. Environmental Science and Pollution Research, 27, 14044–14057. https://doi.org/10.1007/s11356-020-07756-x
Akpomie, K. G., & Dawodu, F. A. (2016). Acid-modified montmorillonite for sorption of heavy metals from automobile effluent. Beni-Suef University Journal of Basic and Applied Sciences, 5, 1–12. https://doi.org/10.1016/j.bjbas.2016.01.003
Alexander, J. A., Zaini, M. A. A., Surajudeen, A., Aliyu, E. N. U., & Omezia, A. U. (2019). Surface modification of low-cost bentonite adsorbents-A review. Particulate Science and Technology, 37(5), 538–549. https://doi.org/10.1080/02726351.2018.1438548
Ali, I. H., Al Mesfer, M. K., Khan, M. I., Danish, M., & Alghamdi, M. M. (2019). Exploring adsorption process of lead(II) and chromium(VI) ions from aqueous solutions on acid activated carbon prepared from juniperus procera leaves. Processes, 7, 217. https://doi.org/10.3390/pr7040217
ASTM 837 C (2000). Test Method for Methylene Blue Index of Clay.
Auerbach, S. M., Carrado, K. A., & Dutta, P. K. (2004). Handbook of Layered Materials, 1st edition. Boca Raton, 664 p. https://doi.org/10.1201/9780203021354d.
Azizian, S. (2004). Kinetic models of sorption: A theoretical analysis. Journal of Colloid and Interface Science, 276(1), 47–52. https://doi.org/10.1016/j.jcis.2004.03.048
Benjelloun, M., Miyah, Y., Akdemir Evrendilek, G., Zerrouq, F., & Lairini, S. (2021). Recent advabces in adsorption kinetic models: Their application to dye types. Arabian Journal of Chemistry, 14(4), 103031. https://doi.org/10.1016/j.arabjc.2021.103031
Bhattacharya, S. (2021). Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. Response Surface Methodology in Engineering Science. https://doi.org/10.5772/intechopen.95835
Birhanu, Y., Leta, S., & Adam, G. (2020). Removal of chromium from synthetic wastewater by adsorption onto Ethiopian low-cost Odaracha adsorbent. Applied Water Science, 10, 227. https://doi.org/10.1007/s13201-020-01310-3
Çinku, K., & Baysal, B. (2014). Investigation of adsorption behavior of phosphonium salts onto Na-montmorillonite. Physicochemical Problems of Mineral Processing, 50(2), 417–432. https://doi.org/10.5277/ppmp140201
Dim, P. E., Mustapha, L. S., Termtanun, M., & Okafor, J. O. (2021). Adsorption of chromium (VI) and iron (III) ions onto acid-modified kaolinite: Isotherm, kinetics and thermodynamics studies. Arabian Journal of Chemistry, 14, 103064. https://doi.org/10.1016/j.arabjc.2021.103064
Elfadli, A. M., Zeid, I. F., Yehia, F. Z., Abouelela, M. M., & Rabie, A. M. (2017). Production of aromatic hydrocarbons from catalytic pyrolysis of lignin over acid activated bentonite clay. Fuel Processing Technology, 163, 1–7. https://doi.org/10.1016/j.fuproc.2017.03.033
Erdoğan Alver, B., Sakızcı, M., & Yörükoğulları, E. (2012). Investigation of Thermal and Gas Adsorption Properties of Acid Activated Bentonite. Süleyman Demirel Üniversitesi, Fen Bilimleri Enstitüsü Dergisi, 16(2), 162–166.
Erdoğan Alver, B. (2018). Hydrogen adsorption on natural and sulphuric acid treated sepiolite and bentonite. International Journal of Hydrogen Energy, 4(3), 831–838. https://doi.org/10.1016/j.ijhydene.2017.10.159
Gutierrez, T. J., Ponce, A. G., & Alvarez, V. A. (2017). Nano-clays from natural and modified montmorillonite with and without added blueberry extract for active and intelligent food nanopackaging materials. Materials Chemistry and Physics, 194, 283–292. https://doi.org/10.1016/j.matchemphys.2017.03.052
Hedberg, Y. S. (2020). Chromium and leather: A review on the chemistry of relevance for allergic contact dermatitis to chromium. Journal of Leather Science and Engineering, 2, 20. https://doi.org/10.1186/s42825-020-00027-y
Ho, Y. S., & McKay, G. (1998). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70, 115–124. https://doi.org/10.1016/S0923-0467(98)00076-1
Hussin, F., Aroua, M. K., & Daud, W. M. A. W. (2011). Textural characteristics, surface chemistry and activation of bleaching earth: A review. Chemical Engineering Journal, 170(1), 90–106. https://doi.org/10.1016/j.cej.2011.03.065
Ibrahim, M. M. (2019). Cr2O3/Al2O3 as adsorbent: Physicochemical properties and adsorption behaviors towards removal of Congo red dye from water. Journal of Environmental Chemical Engineering, 7(1), 102848. https://doi.org/10.1016/j.jece.2018.102848
Jang, E., Pack, S. P., Kim, Il., & Chung, S. A. (2020). Systematic study of hexavalent chromium adsorption and removal from aqueous environments using chemically functionalized amorphous and mesoporous silica nanoparticles. Nature Research Scientific Reports, 10, 5558. https://doi.org/10.1038/s41598-020-61505-1
**g, Q.-X., Chai, L.-Y., Huang, X.-D., Tang, C.-J., Guo, H., & Wang, W. (2017). Behavior of ammonium adsorption by clay mineral halloysite. Trans. Nonferrous Metals Soc. China, 27, 1627–1635. https://doi.org/10.1016/S1003-6326(17)60185-7
Khammour, F., Abdoul-Latif, F. M., Ainane, A., Mohamed, J., & Ainane, T. (2021). Eco-friendly adsorbent from waste of mint: application for the removal of hexavalent chromium. Hindawi Journal of Chemistry, 8848964. https://doi.org/10.1155/2021/8848964
Kütahyalı, C., Sert, Ş, Çetinkaya, B., Yalçıntaş, E., & Acar, M. B. (2012). Biosorption of Ce (III) onto modified Pinus brutia leaf powder using Central Composite Design. Wood Science and Technology, 46(4), 721–736. https://doi.org/10.1016/j.jhazmat.2011.11.047
Noyan, H., Önal, M., & Sarıkaya, Y. (2007). The effect of sulphuric acid activation on the crystallinity, surface area, porosity, surface acidity, and bleaching power of a bentonite. Food Chemistry, 105(1), 156–163. https://doi.org/10.1016/j.foodchem.2007.03.060
Potsi, G., Ladavos, A. K., Petrakis, D., Douvalis, A. P., Sanakis, Y., Katsiotis, M. S., Papavassiliou, G., Alhassan, S., Gournis, D., & Rudolf, P. (2018). Iron-substituted cubic silsesquioxane pillared clays: Synthesis, characterization and acid catalytic activity. Journal of Colloid and Interface Science, 510, 395–406. https://doi.org/10.1016/j.jcis.2017.09.003
Rezende, M. J. C., & Pinto, A. C. (2016). Esterification of fatty acids using acid activated Brazilian smectite natural clay as a catalyst. Renewable Energy, 92, 171–177. https://doi.org/10.1016/j.renene.2016.02.004
Shafiekhania, H., & Barjoizadehb, R. (2019). Modification of activated carbon by ZnCl2, CaCl2, MgCl2 and their applications in removal of nitrate ion from drinking water. Asian Journal of Green Chemistry, 3, 1–12. https://doi.org/10.22034/ajgc.2018.65164
Sheikhi, M., & Rezae, H. (2021). Adsorption of hexavalent chromium ions from aqueous solutions using nano-chitin: Kinetic, isotherms and thermodynamic studies. Water Practice and Technology, 16(2), 436–451. https://doi.org/10.2166/wpt.2021.007
Tamjidi, S., Moghadas, B. K., Esmaeili, H., Khoo, F. S., Gholami, G., & Ghasemi, M. (2021). Improving the surface properties of adsorbents by surfactants and their role in the removal of toxic metals from wastewater: A review study. Process Safety and Environmental Protection, 148, 775–795. https://doi.org/10.1016/j.psep.2021.02.003
Timofeeva, M. N., Panchenko, V. N., Krupskaya, V. V., Gil, A., Miguel, A., & Vicente, M. A. (2017). Effect of nitric acid modification of montmorillonite clay on synthesis of solketal from glycerol and acetone. Catalysis Communications, 90, 65–69. https://doi.org/10.1016/j.catcom.2016.11.020
Vo, T. S., Hossain, M. M., Jeong, H. M., & Kim, K. (2020). Heavy metal removal applications using adsorptive membranes. Nano Convergence, 7(36), 1–26. https://doi.org/10.1186/s40580-020-00245-4
Ye, Z., Yin, X., Chen, L., He, X., Lin, Z., Liu, C., Ning, S., Wang, X., & Wei, Y. (2019). An integrated process for removal and recovery of Cr(VI) from electroplating wastewater by ion exchange and reduction–precipitation based on a silica-supported pyridine resin. Journal of Cleaner Production, 236, 117631. https://doi.org/10.1016/j.jclepro.2019.117631
Zheng, C., Yang, Z., Si, M., Zhu, F., Yang, W., Zhao, F., & Shi, Y. (2021). Application of biochars in the remediation of chromium contamination: Fabrication, mechanisms, and interfering species. Journal of Hazardous Materials, 407, 124376. https://doi.org/10.1016/j.jhazmat.2020.124376
Acknowledgements
The writers are grateful for the valuable contribution to Dr Mehmet Bahadir ACAR and Dr Berkan CETINKAYA. The authors would like to thank Ceylan Mineral Madencilik San. ve Tic. A.S. for supplying and allowing the use of bentonites. The writers would like to thank Gazi University Scientific Research Projects Department for funding the 05/2015-14 coded project including this study. Also, the authors would like to thank Gazi University Academic Writing Application and Research Center (Certificate Number: 30.11.2021/0108).
Author information
Authors and Affiliations
Contributions
BCA: Conceptualization, Methodology, Investigation, Writing—Original Draft, Project administration.
ZY: Methodology, Investigation, Resources, Funding acquisition, Supervision.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics Approval
This article contains no studies with human participants or animals performed by any authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cinar Acar, B., Yuksekdag, Z. Investigation of Chromium (III) Adsorption on Acid-Treated Bentonite Evaluation of Kinetic/Thermodynamic Data. Water Air Soil Pollut 234, 716 (2023). https://doi.org/10.1007/s11270-023-06727-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06727-5