Abstract
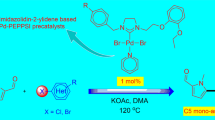
Two 1,2,3-triazole-derived monocationic salts 3 and 4 bearing N-aryl wingtips were prepared using copper-catalyzed “click” reactions followed by alkylations with iodomethane. Employing a silver–carbene transfer method, two dinuclear palladium(II) complexes of triazolin-5-ylidenes (5/6) were obtained, the former of which has been reported previously. Bridge-cleavage reaction of 5 as a representative with PPh3 yielded cis-configured mesoionic carbene/phosphine hybrid complex cis-7 and homoleptic bis(phosphine) complex 8, suggesting the presence of a ligand exchange process. In contrast, bridge breakages of 5/6 with pyridine cleanly afforded PEPPSI-type complexes 9 and 10 in near quantitative yields. Finally, all complexes were exploited to catalyze Mizoroki–Heck coupling reactions with aryl bromides as the substrates, and PEPPSI-type complex 9 was found to be the best performer generally giving good to excellent yields.








Similar content being viewed by others
Notes
See [18].
See [28].
The aryl C–Ha proton expectedly give a doublet with a small coupling constant of ~ 2–3 Hz, due to (i) the absence of protons attached to the Cortho atoms and (ii) the splitting by C–H protons of the Cmeta atoms. In our case, a singlet was observed probably due to the low resolution of NMR spectrometer.
For a review on agostic and anagostic interactions, see [46].
The term “transphobia effect” was first coined by Vicente and Jones’s groups. For this paper, see [49].
For a paper on the definition of π–π stacking, see [52].
See [53].
See [54].
References
Hahn FE, Jahnke MC (2008) Angew Chem Int Ed 47:3122–3172
Gu S, Chen C, Chen W (2011) Curr Org Chem 15:3291–3308
Díez-González S, Marion N, Nolan SP (2009) Chem Rev 109:3612–3676
Normand AT, Cavell KJ (2008) Eur J Inorg Chem 18:2781–2800
Schaper LA, Hock SJ, Herrmann WA, Kühn FE (2013) Angew Chem Int Ed 52:270–289
Peris E (2017) Chem Rev. https://doi.org/10.1021/acs.chemrev.6b00695
Hameury S, de Frémont P, Braunstein P (2017) Chem Soc Rev 46:632–733
Janssen-Müller D, Schlepphorst C, Glorius F (2017) Chem Soc Rev 46:4845–4854
Kantchev EAB, O’Brien CJ, Organ MG (2007) Angew Chem Int Ed 46:2768–2813
Fortman GC, Nolan SP (2011) Chem Soc Rev 40:5151–5169
Herrmann WA, Böhm VPW, Gstöttmayr CWK, Grosche M, Reisinger CP, Weskamp T (2001) J Organomet Chem 617–618:616–628
Yang J (2017) Appl Organomet Chem 31:3734–3739
Farmer JL, Pompeo M, Lough AJ, Organ MG (2014) Chem Eur J 20:15790–15798
Huynh HV, Han Y, Ho JHH, Tan GK (2006) Organometallics 25:3267–3274
Huynh HV, Han Y, Jothibasu R, Yang JA (2009) Organometallics 28:5395–5404
Valente C, Pompeo M, Sayah M, Organ MG (2014) Org Process Res Dev 18:180–190
Valente C, Çalimsiz S, Hoi KH, Mallik D, Sayah M, Organ MG (2012) Angew Chem Int Ed 51:3314–3332
Arduengo AJ III, Harlow RL, Kline M (1991) J Am Chem Soc 113:361–363
Crabtree RH (2013) Coord Chem Rev 257:755–766
Shuster O, Yang L, Raubenheimer HG, Albrecht M (2009) Chem Rev 109:3445–3478
Albrecht M (2008) Chem Commun 31:3601–3610
Arnold PL, Pearson S (2007) Coord Chem Rev 251:596–609
Krüger A, Albrecht M (2011) Aust J Chem 64:1113–1117
Poulain A, Iglesias M, Albrecht M (2011) Curr Org Chem 15:3325–3336
Tolman CA (1970) J Am Chem Soc 92:2953–2956
Dröge T, Glorius F (2010) Angew Chem Int Ed 49:6940–6952
Nelson DJ, Nolan SP (2013) Chem Soc Rev 42:6723–6753
Teng Q, Huynh HV (2017) Dalton Trans 46:614–627
Mathew P, Neels A, Albrecht M (2008) J Am Chem Soc 130:13534–13535
Donnelly KF, Petronilho A, Albrecht M (2013) Chem Commun 49:1145–1159
Crowley JD, Lee AL, Kilpin KJ (2011) Aust J Chem 64:1118–1132
Wang C, Ikhlef D, Kahlal S, Saillardb J, Astruc D (2016) Coord Chem Rev 316:1–20
Liang L, Astruc D (2011) Coord Chem Rev 255:2933–2945
Meldal M, Tornøe CW (2008) Chem Rev 108:2952–3015
Canseco-Gonzalez D, Gniewek A, Szulmanowicz M, Müller-Bunz H, Trzeciak AM, Albrecht M (2012) Chem Eur J 18:6055–6062
Huang J, Hong JT, Hong SH (2012) Eur J Org Chem 33:6630–6635
Mendoza-Espinosa D, Gonzáez-Olvera R, Osornio C, Negrón-Silva GE, Álvarez-Hernández A, Bautista-Hernández CI, Suárez-Castillo OR (2015) J Organomet Chem 803:142–149
Dasgupta A, Ramkumar V, Sankararaman S (2016) Eur J Org Chem 28:4817–4823
Sureshbabu B, Ramkumar V, Sankararaman S (2015) J Organomet Chem 799–800:232–238
Egbert JD, Cazin CSJSP, Nolan SP (2013) Catal. Sci Technol 3:912–926
Díez-González S (2011) Catal. Sci Technol 1:166–178
Díez-González S, Correa A, Cavallo L, Nolan SP (2006) Chem Eur J 12:7558–7564
Poulain A, Canseco-Gonzalez D, Hynes-Roche R, Müller-Bunz H, Schuster O, Stoeckli-Evans H, Neels A, Albrecht M (2011) Organometallics 30:1021–1029
Sakamoto T, Uchiyama D, Kondo Y, Yamanaka H (1993) Heterocycles 35:1273–1278
Fletcher JT, Keeney ME, Walz SE (2010) Synthesis 19:3339–3345
Brookhart M, Green MLH, Parkin G (2007) PNAS 104:6908–6914
Kubota M, Ohba S, Saito Y (1991) Acta Crystallogr Sect C Cryst Struct Commun 47:1727–1729
Cave GWV, Errington W, Rourke JP (1999) Acta Crystallogr Sect C Cryst Struct Commun 55:320–322
Vicente J, Arcas A, Bautista D, Jones PG (1997) Organometallics 16:2127–2138
Dasgupta A, Ramkumar V, Sankararaman S (2015) RSC Adv 5:21558–21561
Modak S, Gangwar MK, Rao MN, Madasu M, Kalita AC, Dorcet V, Shejale MA, Butcher RJ, Ghosh P (2015) Dalton Trans 44:17617–17628
Janiak C (2000) J Chem Soc, Dalton Trans 0:3885–3896
Herrmann WA, Böhm VPW, Gstöttmayr CWK, Grosche M, Reisinger CP, Weskamp T (2001) J Organomet Chem 617–618:616–628
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339–341
Acknowledgements
This work is financially supported by Capacity Building for Sci-Tech Innovation-Fundamental Scientific Research Funds (Grant No. 025185305000/208) and Department of Education of Guangdong Province (Grant No. 2016KCXTD005, 2017KQNCX204).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Yan, X., Zhang, B. et al. Mono- and dinuclear palladium(II) complexes incorporating 1,2,3-triazole-derived mesoionic carbenes: syntheses, solid-state structures and catalytic applications. Transit Met Chem 44, 39–48 (2019). https://doi.org/10.1007/s11243-018-0267-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0267-8