Abstract
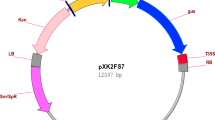
Portulaca oleracea is an important medicinal plant, which is a source of pharmacologically active molecules such as β-Carotene, ascorbic acid, and Omega-3 fatty acids. The present research focuses on the development of an efficient protocol for micropropagation and Agrobacterium-mediated genetic transformation of P. oleracea. Callus induction, somatic embryogenesis, and plant regeneration from stem and leaf explants were investigated at various concentrations of kinetin (Kin) and 6-Benzylaminopurine (BAP) alone or in combination with indole-3-acetic acid, 1-Naphthaleneacetic acid and 2,4-Dichlorophenoxyacetic acid (2,4-D). Direct differentiation of somatic embryos from leaf explants occurred on the MS medium supplemented with 1.5 mg/L BAP under dark conditions. The embryos were transferred to the same medium without growth regulators under 16 h light/8 h dark cycles. In this medium, germinated somatic embryos rapidly developed into healthy plantlets with shoots and roots. Several parameters such as pre-culture of explants, co-cultivation period, wounding of explants, type of explants and bacterial strains were studied to optimize transformation efficiency. Different kanamycin concentrations were assessed for the selection of transgenic plants. Agrobacterium tumefaciens strains LBA4404 and GV3101, harbouring the GUS gene on pBI121 binary vector, were used for plant transformation and strain LBA4404 was found to be more efficient. The results indicated that use of leaf as explant, pre-culture of explants for 7 days, co-cultivation period for 4 days at 25 ± 2 °C and wounding of leaf explants produced the best transformation results. Expression, integration and inheritance of GUS reporter gene were confirmed by histochemical and molecular analyses.






Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- AS:
-
Acetosyringone
- BAP:
-
6-Benzylaminopurine
- CaMV35S:
-
Cauliflower mosaic virus 35S
- Cx:
-
Cefotaxime
- IAA:
-
Indole-3-acetic acid
- Kin:
-
Kinetin
- Km:
-
Kanamycin
- MS:
-
Murashige and Skoog
- NAA:
-
1-Naphthaleneacetic acid
- X-Gluc:
-
5-Bromo-4-chloro-3-indolyl β-D-glucuronide
References
Alabadí D, Blázquez MA (2009) Molecular interactions between light and hormone signaling to control plant growth. Plant Mol Biol 69(4):409
An G (1985) High efficiency transformation of cultured tobacco cells. Plant Physiol 79(2):568–570
Azad M, Yokota S, Begum F, Yoshizawa N (2009) Plant regeneration through somatic embryogenesis of a medicinal plant, Phellodendron amurense Rupr. In Vitro Cell Dev Biol 45(4):441–449
Bakhshaie M, Babalar M, Mirmasoumi M, Khalighi A (2010) Somatic embryogenesis and plant regeneration of Lilium ledebourii (Baker) Boiss., an endangered species. Plant Cell Tissue Organ Cult 102(2):229–235. https://doi.org/10.1007/s11240-010-9726-4
Chandore A, Nimbalkar M, Gurav R, Bapat V, Yadav S (2010) A protocol for multiplication and restoration of Ceropegia fantastica Sedgw.: a critically endangered plant species. Curr Sci 2010:1593–1596
Chée RP, Cantliffe DJ (1989) Inhibition of somatic embryogenesis in response to 2,3,5-triiodobenzoic acid and 2,4-dichlorophenoxyacetic acid in Ipomoea batatas (L.) Lam. cultured in vitro. J Plant Physiol 135(4):398–403. https://doi.org/10.1016/S0176-1617(89)80094-X
Cheng ZJ, Zhu SS, Gao XQ, Zhang XS (2010) Cytokinin and auxin regulates WUS induction and inflorescence regeneration in vitro in Arabidopsis. Plant Cell Rep 29(8):927–933. https://doi.org/10.1007/s00299-010-0879-8
Chitra Devi B, Narmathabai V (2011) Somatic embryogenesis in the medicinal legume Desmodium motorium (Houtt.) Merr. Plant Cell Tissue Organ Cult 106(3):409–418. https://doi.org/10.1007/s11240-011-9937-3
Chung J-P, Huang C-Y, Dai T-E (2010) Spectral effects on embryogenesis and plantlet growth of Oncidium ‘Gower Ramsey’. Sci Hortic 124(4):511–516
Collado R, Bermúdez-Caraballoso I, García L, Veitía N, Torres D, Romero C, Martirena-Ramírez A, Angenon G (2015) Agrobacterium-mediated transformation of Phaseolus vulgaris L. using indirect organogenesis. Sci Hortic 195:89–100
Dai H, Li W, Han G, Yang Y, Ma Y, Li H, Zhang Z (2013) Development of a seedling clone with high regeneration capacity and susceptibility to Agrobacterium in apple. Sci Hortic 164:202–208
Delfan-Hosseini S, Nayebzadeh K, Mirmoghtadaie L, Kavosi M, Hosseini SM (2017) Effect of extraction process on composition, oxidative stability and rheological properties of purslane seed oil. Food Chem 222:61–66
Deo PC, Tyagi AP, Taylor M, Harding RM, Becker DK (2010) Factors affecting somatic embryogenesis and transformation in modern plant breeding. S Pac J Nat Appl Sci 28(1):27–40
Dhar U, Joshi M (2005) Efficient plant regeneration protocol through callus for Saussurea obvallata (DC.) Edgew.(Asteraceae): effect of explant type, age and plant growth regulators. Plant Cell Rep 24(4):195–200
Dibax R, Eisfeld CDL, Cuquel FL, Koehler H, Quoirin M (2005) Plant regeneration from cotyledonary explants of Eucalyptus camaldulensis. Sci Agricola 62(4):406–412
Dutt M, Grosser J (2009) Evaluation of parameters affecting Agrobacterium-mediated transformation of citrus. Plant Cell Tissue Organ Cult 98(3):331–340
Farhadi N, Panahandeh J, Azar AM, Salte SA (2017) Effects of explant type, growth regulators and light intensity on callus induction and plant regeneration in four ecotypes of Persian shallot (Allium hirtifolium). Sci Hortic 218:80–86
Feher A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74(3):201–228
Filippov M, Miroshnichenko D, Vernikovskaya D, Dolgov S (2006) The effect of auxins, time exposure to auxin and genotypes on somatic embryogenesis from mature embryos of wheat. Plant Cell Tissue Organ Cult 84(2):213–222. https://doi.org/10.1007/s11240-005-9026-6
Fujimura T, Komamine A (1979) Involvement of endogenous auxin in somatic embryogenesis in a carrot cell suspension culture. Z Pflanzenphysiol 95(1):13–19. https://doi.org/10.1016/S0044-328X(79)80023-9
Gaj MD (2004) Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul 43(1):27–47
Gomes F, Simoes M, Lopes ML, Canhoto JM (2010) Effect of plant growth regulators and genotype on the micropropagation of adult trees of Arbutus unedo L.(strawberry tree). New Biotechnol 27(6):882–892
Gutiérrez MA, Luth D, Moore GA (1997) Factors affecting Agrobacterium-mediated transformation in Citrus and production of sour orange (Citrus aurantium L.) plants expressing the coat protein gene of citrus tristeza virus. Plant Cell Rep 16(11):745–753. https://doi.org/10.1007/s002990050313
Hooley R (1999) A role for G proteins in plant hormone signalling? Plant Physiol Biochem 37(5):393–402. https://doi.org/10.1016/S0981-9428(99)80045-X
Huang P, Xu M, **a L, Qing Z, Tang Q, Liu W, Zeng J (2017) Establishment of an efficient Agrobacterium-mediated genetic transformation method in Macleaya cordata. Sci Hortic 226:302–306
Japelaghi RH, Haddad R, Garoosi G-A (2011) Rapid and efficient isolation of high quality nucleic acids from plant tissues rich in polyphenols and polysaccharides. Mol Biotechnol 49(2):129–137
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5(4):387–405
Jha TB, Mukherjee P, Datta MM (2007) Somatic embryogenesis in Jatropha curcas Linn., an important biofuel plant. Plant Biotechnol Rep 1(3):135–140. https://doi.org/10.1007/s11816-007-0027-2
Jiang H, Doerge R, Gelvin SB (2003) Transfer of T-DNA and Vir proteins to plant cells by Agrobacterium tumefaciens induces expression of host genes involved in mediating transformation and suppresses host defense gene expression. Plant J 35(2):219–236
Jiménez VM (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47(2–3):91–110
Khan S, Fahim N, Singh P, Rahman LU (2015) Agrobacterium tumefaciens mediated genetic transformation of Ocimum gratissimum: a medicinally important crop. Ind Crops Prod 71:138–146
Kim HS, Zhang G, Juvik JA, Widholm JM (2010) Miscanthus × giganteus plant regeneration: effect of callus types, ages and culture methods on regeneration competence. Gcb Bioenergy 2(4):192–200
Lu M-C (2005) Micropropagation of Vitis thunbergii Sieb. et Zucc., a medicinal herb, through high-frequency shoot tip culture. Sci Hortic 107(1):64–69. https://doi.org/10.1016/j.scienta.2005.05.014
Lu G, Zou Q, Guo D, Zhuang X, Yu X, **ang X, Cao J (2007) Agrobacterium tumefaciens-mediated transformation of Narcissus tazzeta var. chinensis. Plant Cell Rep 26(9):1585–1593
Michniewicz M, Brewer PB, Friml J (2007) Polar auxin transport and asymmetric auxin distribution. Arabidopsis Book 5:e0108
Moghadam YA, Piri K, Bahramnejad B, Habibi P (2011) Hairy roots induction in purslane (Portulaca oleracea Linn.) using Agrobacterium rhizogenes. Plant Med 77(12):PB24
Mohan C, Naresh B, Kumar BK, Reddy V, Manjula P, Keerthi B, Sreekanth D, Manzelat SF, Cherku PD (2017) Micropropagation studies and phytochemical analysis of the endangered tree Commiphora wightii. J Appl Res Med Aromat Plants 6:70–79. https://doi.org/10.1016/j.jarmap.2017.02.004
Mukeshimana G, Ma Y, Walworth AE, Song G-Q, Kelly JD (2013) Factors influencing regeneration and Agrobacterium tumefaciens-mediated transformation of common bean (Phaseolus vulgaris L.). Plant Biotechnol Rep 7(1):59–70. https://doi.org/10.1007/s11816-012-0237-0
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Naeem F, Khan SH (2013) Purslane (Portulaca oleracea L.) as phytogenic substance—a review. J Herbs Spices Med Plants 19(3):216–232
Nato A, Fresneau C, Moursalimova N, De Buyser J, Lavergne D, Henry Y (2000) Expression of auxin and light-regulated arrestin-like proteins, G proteins and nucleoside diphosphate kinase during induction and development of wheat somatic embryos. Plant Physiol Biochem 38(6):483–490. https://doi.org/10.1016/S0981-9428(00)00769-5
Parimalan R, Venugopalan A, Giridhar P, Ravishankar G (2011) Somatic embryogenesis and Agrobacterium-mediated transformation in Bixa orellana L. Plant Cell Tissue Organ Cult 105(3):317–328
Petropoulos S, Karkanis A, Martins N, Ferreira IC (2016) Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci Technol 55:1–10
Pozueta-Romero J, Houlné G, Cañas L, Schantz R, Chamarro J (2001) Enhanced regeneration of tomato and pepper seedling explants for Agrobacterium-mediated transformation. Plant Cell Tissue Organ Culture 67(2):173–180
Prakash M, Gurumurthi K (2010) Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult 100(1):13
Quiala E, Cañal M-J, Meijón M, Rodríguez R, Chávez M, Valledor L, de Feria M, Barbón R (2012) Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments. Plant Cell Tissue Organ Cult 109(2):223–234. https://doi.org/10.1007/s11240-011-0088-3
Ramage CM, Williams RR (2004) Cytokinin-induced abnormal shoot organogenesis is associated with elevated Knotted1-type homeobox gene expression in tobacco. Plant cell Rep 22(12):919–924
Rossin CB, Rey MEC (2011) Effect of explant source and auxins on somatic embryogenesis of selected cassava (Manihot esculenta Crantz) cultivars. S Afr J Bot 77(1):59–65. https://doi.org/10.1016/j.sajb.2010.05.007
Rout GR, Samantaray S, Das P (2000) In vitro manipulation and propagation of medicinal plants. Biotechnol Adv 18(2):91–120. https://doi.org/10.1016/S0734-9750(99)00026-9
Safdari Y, Kazemitabar S (2009) Plant tissue culture study on two different races of purslane (Portulaca oleracea L.). Afr J Biotechnol 8(21):5906–5912
Sahai A, Shahzad A, Sharma S (2010) Histology of organogenesis and somatic embryogenesis in excised root cultures of an endangered species Tylophora indica (Asclepiadaceae). Aust J Bot 58(3):198–205
Saini R, Jaiwal P (2007) Agrobacterium tumefaciens-mediated transformation of blackgram: an assessment of factors influencing the efficiency of uidA gene transfer. Biol Plant 51(1):69–74
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual, Cold Spring Laboratory. Cold Spring Harbor, NY. VIII. Appendix A. pBIND Vector Sequence (continued) A. pBIND Vector Sequence (continued) B. pBIND Vector Restriction Sites Enzyme# of Sites. Location Dra I 4(1857):4877
Sharma M, Kothari-Chajer A, Jagga-Chugh S, Kothari S (2011a) Factors influencing Agrobacterium tumefaciens-mediated genetic transformation of Eleusine coracana (L.) Gaertn. Plant Cell, Tissue Organ Cult 105(1):93–104
Sharma MM, Singh A, Verma RN, Ali DZ, Batra A (2011b) Influence of PGRS for the in vitro plant regeneration and flowering in Portulaca oleracea L.: a medicinal and ornamental plant. Int J Bot 7(1):103–107
Shekhawat MS, Kannan N, Manokari M (2015) Propagation OF Portulaca oleracea L. in liquid medium: implications of plant growth regulators in culture. J Microbiol Biotechnol Food Sci 4(4):332
Shrawat AK, Lörz H (2006) Agrobacterium-mediated transformation of cereals: a promising approach crossing barriers. Plant Biotechnol J 4(6):575–603
Stachel SE, Messens E, Van Montagu M, Zambryski P (1985) Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318(6047):624
Su YH, Zhao XY, Liu YB, Zhang CL, O’Neill SD, Zhang XS (2009) Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J 59(3):448–460
Su Y-H, Liu Y-B, Zhang X-S (2011) Auxin–cytokinin interaction regulates meristem development. Mol Plant 4(4):616–625. https://doi.org/10.1093/mp/ssr007
Su YH, Liu YB, Bai B, Zhang XS (2015) Establishment of embryonic shoot–root axis is involved in auxin and cytokinin response during Arabidopsis somatic embryogenesis. Front Plant Sci 5:792
Suzuki RM, Kerbauy GB, Zaffari GR (2004) Endogenous hormonal levels and growth of dark-incubated shoots of Catasetum fimbriatum. J Plant Physiol 161(8):929–935
Swamy MK, Mohanty SK, Anuradha M (2014) The effect of plant growth regulators and natural supplements on in vitro propagation of Pogostemon cablin Benth. J Crop Sci Biotechnol 17(2):71–78
Thorpe TA (2007) History of plant tissue culture. Mol Biotechnol 37(2):169–180. https://doi.org/10.1007/s12033-007-0031-3
Tian Q, Reed JW (2001) Molecular links between light and auxin signaling pathways. J Plant Growth Regul 20(3):274–280. https://doi.org/10.1007/s003440010022
Tiwari V, Singh BD, Tiwari KN (1998) Shoot regeneration and somatic embryogenesis from different explants of Brahmi [Bacopa monniera (L.) Wettst.]. Plant Cell Rep 17(6–7):538–543
Tzfira T, Vaidya M, Citovsky V (2002) Increasing plant susceptibility to Agrobacterium infection by overexpression of the Arabidopsis nuclear protein VIP1. Proc Natl Acad Sci USA 99(16):10435–10440
Uddin MK, Juraimi AS, Hossain MS, Nahar MAU, Ali ME, Rahman M (2014) Purslane weed (Portulaca oleracea): a prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci World J 2014:1–6. https://doi.org/10.1155/2014/951019
Viktor Nørgaard J, Krogstrup P (1991) Cytokinin induced somatic embryogenesis from immature embryos of Abies nordmanniana Lk. Plant Cell Rep 9(9):509–513. https://doi.org/10.1007/bf00232107
Witbooi H, Okem A, Makunga N, Kambizi L (2017) Micropropagation and secondary metabolites in Agathosma betulina (Berg.). S Afr J Bot 111:283–290
Yan M-M, Xu C, Kim C-H, Um Y-C, Bah AA, Guo D-P (2009) Effects of explant type, culture media and growth regulators on callus induction and plant regeneration of Chinese jiaotou (Allium chinense). Sci Hortic 123(1):124–128. https://doi.org/10.1016/j.scienta.2009.07.021
Yang Y, Bao M, Liu G (2010) Factors affecting Agrobacterium-mediated genetic transformation of embryogenic callus of Parthenocissus tricuspidata Planch. Plant Cell Tissue Organ Cult 102(3):373–380
Zelená E (2000) The effect of light on metabolism of IAA in maize seedlings. Plant Growth Regul 30(1):23–29
Zhang B-H, Feng R, Liu F, Zhou D-Y, Wang Q-L (2001) Direct somatic embryogenesis and plant regeneration from cotton (Gossypium hirsutum L.) explants. Israel J Plant Sci 49(3):193–196
Zhou Y-X, **n H-L, Rahman K, Wang S-J, Peng C, Zhang H (2015) Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. BioMed Res Int 2015:1–11. https://doi.org/10.1155/2015/925631
Ziemienowicz A (2014) Agrobacterium-mediated plant transformation: factors, applications and recent advances. Biocatal Agric Biotechnol 3(4):95–102
Acknowledgements
This paper is part of the PhD dissertation of the first author. These experiments were conducted at the Molecular Biology Laboratory and Tissue Culture Laboratory of Faculty of Agricultural Sciences and Natural Resources, Imam Khomeini International University, Qazvin, Iran. The authors would like to thank PhD Students; Reza Heidari Japelaghi and Meysam Bastami for their technical assistance.
Author information
Authors and Affiliations
Contributions
BS: Performance of experiments, collection and analysis of data and preparation of manuscript; RH: Corresponding author, chief scientist, assistance to data collection and analysis; financial supports, experimental design. MB: Project academic advisor, preparation of plant materials and assistance to experimental design.
Corresponding author
Additional information
Communicated by Danny Geelen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sedaghati, B., Haddad, R. & Bandehpour, M. Efficient plant regeneration and Agrobacterium-mediated transformation via somatic embryogenesis in purslane (Portulaca oleracea L.): an important medicinal plant. Plant Cell Tiss Organ Cult 136, 231–245 (2019). https://doi.org/10.1007/s11240-018-1509-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1509-3