Abstract
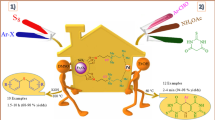
Magnetic nano-Fe3O4 based on adenine was synthesized with surface high-density functional group, namely SO3H, so that it is a novel Brønsted acid nanomagnetic catalyst, and acts as a solid heterogeneous catalyst. Also in this catalyst, the surface of the Fe3O4 nanoparticles was protected using carbon quantum dots (CQDs). The as-synthesized catalyst was characterized using different techniques, including Fourier transform infrared spectroscopy (FT-IR), X-ray powder diffraction (XRD), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), dynamic light scattering (DLS), energy-dispersive X-ray spectroscopy (EDX), energy-dispersive X-ray spectroscopy map** (EDX map**), vibrating sample magnetometer (VSM), thermal gravimetric analysis (TGA) and differential thermal analysis (DTA). The activity of the as-synthesized catalyst was evaluated in the synthesis of 5-amino-1, 3-diphenyl-1H-pyrazole 4-carbonitril and pyrano[2,3-c] pyrazole derivatives. The proposed method has important features which include solvent-free, mild, metal-free, high yield, shorter reaction time, easy recovery of catalyst and easy workup procedure. The effect of temperature and the amount of the catalyst (optimum reaction condition) were determined using a systematic approach, namely the design of experiment (DoE).





















Similar content being viewed by others
References
R. Singh, R.M. Kissling, M.A. Letellier, S.P. Nolan, J. Org. Chem. 69, 209 (2004)
S.H. Banakar, M.G. Dekamin, A. Yaghoubi, New J Chem. 42, 14246 (2018)
S.A. Salem, A. Khazaei, J. Y Seyf, N. Sarmasti and M. M. Gilan, Polycycl Aromat Compd., 41 (2019).
X. Liu, C. Yang, B. Zheng, J. Dai, L. Yan, Zh. Zhuang, J. Du, Y. Guo, D. **ao, Sens. Actuators B Chem. 255, 572 (2018)
T. Akbarpour, A. Khazaei, J. Yousefi Seyf, N. Sarmasti, Appl. Organomet. Chem. 10, e6361 (2021)
N. Sarmasti, J. Yousefi Seyf, A. Khazaei, Arab. J. Chem. 3, 103026 (2021)
A. Azarifar, R. Nejat-Yami, M. Al Kobaisi, D. Azarifar, J. Iran. Chem. Soc. 3, 439 (2013)
H. Faroughi Niya, N. Hazeri, M.T. Maghsoodlou, Appl. Organomet. Chem. 4, e5472 (2020)
Z. Alirezvani, M.G. Dekamin, E. Valiey, ACS Omega 24, 20618 (2019)
A. Khazaei, N. Sarmasti, J. Yousefi Seyf, M. Tavasoli, RSC Adv. 123, 101268 (2015)
A. Maleki, V. Eskandarpour, J. Iran. Chem. Soc. 16, 1459 (2019)
H.F. Rizk, S.A. Ibrahim, M.A. El-Borai, Dyes Pigm. 112, 86 (2015)
M.A. Elsherif, A.S. Hassan, G.O. Moustafa, H.M. Awad, N.M. Morsy, J. Appl. Pharm. Sci. 10, 37 (2020)
S. Ananou, M. Maqueda, M. Martínez-Bueno, A. Gálvez, E. Valdivia, Lett. Appl. Microbiol. 1, 19 (2007)
J. Cleveland, T.J. Montville, I.F. Nes, M.L. Chikindas, Int. J. Food Microbiol. 1, 1 (2001)
F. Leroy, L. De Vuyst, Appl. Environ. Microbiol. 67, 4407 (2001)
V. Bali, P.S. Panesar, M.B. Bera, J. Microbiol. 1, 113 (2011)
B.G. Shaw, DCh. Harding, J. Appl. Microbiol. 56, 25 (1984)
S. Rodgers, K. Kailasapathy, J. Cox, P. Peiris, Food Serv. Technol. 2, 59 (2002)
A. R. Moosavi-Zare, H. Goudarziafshar, M. Yadollahi, and Z. Jalilian, Polycycl Aromat Compd., (2022) 1.
R. Singh, Res. Chem. Intermed. 45, 4531 (2019)
H.M. Elnagdy, D. Sarma, ChemistrySelect 4, 783 (2019)
A.R. Moosavi-Zare, M. Rezaei-Gohar, M. Tavasoli, H. Goudarziafshar, Res. Chem. Intermed. 47, 2689 (2021)
Z. Abshirini, N. Lotfifar, A. Zare, Org. Prep. Proced. Int. 52, 428 (2020)
I. Yavari, J. Sheykhahmadi, Mol. Divers. (2021) 1.
N.R. Mohamed, N.Y. Khaireldin, A.F. Fahmyb, A.A.F. El-Sayeda, Der Pharma Chem. 2, 400 (2010)
T. Akbarpour, J. Yousefi Seyf, A. Khazaei, and N. Sarmasti, Polycycl Aromat Compd. (2021) 1.
A. Siddekha, A. Nizam, M.A. Pasha, Spectrochim. Acta A. Mol. Biomol. Spectrosc. 81, 431 (2011)
S. Bahrami, F. Hassanzadeh-Afruzi, A. Maleki, Appl. Organomet. Chem. 11, e5949 (2020)
Z.K. Jaberi, M.M.R. Shams, B. Pooladian, Acta. Chim. Slov. 60, 105 (2013)
A. Maleki, Polycycl Aromat Compd. 38, 402 (2018)
M. Kamalzare, M.R. Ahghari, M. Bayat, A. Maleki, Sci. Rep. 11, 1 (2021)
Z.K. Jaberi, M.M.R. Shams, B. Pooladian, Acta Chim. Slov. 1, 105 (2013)
M. Soleimani, A. Khazaei, N. Sarmasti, T. Akbarpour, J. Iran. Chem. Soc., (2021) 1.
M. Solgi, A. Khazaei, T. Akbarpour, Res. Chem. Intermed. 48, 6 (2022) 1.
M.A. Ashraf, Z. Liu, Ch. Li, D. Zhang, Synth. Commun. 50, 2906 (2020)
D. Prabu, P. Senthil Kumar, S. Indraganti, S. Sathish, J. Aravind Kumar, K. Vijai Anand, Sustainability. 14, 2290 (2022)
A. P. Kumar, D. Bilehal, T. Desalegn, S. Kumar, F. Ahmed, H. C. Murthy, and Y. I. Lee, Adsorp. Sci. Technol. (2022) 2022.
S. Stone, T. Wang, J. Liang, J. Cochran, J. Green, W. Gu, Org. Biomol. Chem. 13, 10471 (2015)
P.M. Murray, F. Bellany, L. Benhamou, D.K. Bučar, A.B. Tabor, and T. D. Sheppard Org Biomol Chem. 14, 2373 (2016)
Acknowledgements
The authors would like to appreciate the Research Council of Bu Ali Sina University for providing the facilities and financial support for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmadi, E., Khazaei, A. & Akbarpour, T. [Fe3O4@CQD@Si(OEt)(CH2)3NH@CC@Ad@SO3H]+Cl−: As a new, efficient, magnetically separable and reusable heterogeneous solid acid catalyst for the synthesis of 5-amino-1,3-diphenyl-1H-pyrazole 4-carbonitril and pyrano[2,3-c] pyrazole derivatives. Res Chem Intermed 49, 2099–2122 (2023). https://doi.org/10.1007/s11164-022-04919-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04919-y