Abstract
Entomopathogenic fungi (EPF) are microorganisms that cause fatal diseases of arthropods. The infection process involves several stages that consist of direct contact of the fungus with the surface of the cuticle of the attacked insect. The factors that determine the effectiveness of the infection process include lytic enzymes, secondary metabolites, and adhesins produced by EPF. Because of their high insecticidal effectiveness, these fungi are commonly used as biopesticides in organic farming. As the environment and farmlands are contaminated with many compounds of anthropogenic origin (e.g., pesticides), the effects of these toxic compounds on EPF and the mechanisms that affect their survival in such a toxic environment have been studied in recent years. This review presents information on the capacity of EPF to remove toxic contaminants, including alkylphenols, organotin compounds, synthetic estrogens, pesticides and hydrocarbons. Moreover, these fungi produce numerous secondary metabolites that can be potentially used in medicine or as antimicrobial agents. Despite their huge potential in biocontrol processes, the use of EPF has been underestimated due to a lack of knowledge on their abilities. In our work, we have presented the available data on the possibilities of the additional and unconventional use of these microorganisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
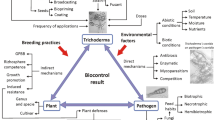
Entomopathogenic fungi (EPF) are bioinsecticides with an ability to infect and kill arthropods. Although they are mainly isolated from arthropod carcasses, their natural habitat is soil (Behie and Bidochka 2014). These fungi are categorized in six classes: Oomycetes, Chytridiomycota, Microsporidia, Entomophtoromycota, Basidiomycota, and the most common Ascomycota. The main function of EPF in the environment is the biocontrol of the insect population. The literature data provide information on the infectious properties of EPF, the infectious process (Skinner et al. 2014; Lacey et al. 2015; Mascarin and Jaronski 2016), and the use of these microorganisms in commercial preparations as biopesticides (Jaihan et al. 2016; Ríos-Moreno et al. 2016).
In the last few decades, the constantly increasing environmental pollution is a serious concern. Every day substances are being introduced in the environment that have an adverse effect on the balance of the ecosystem and living organisms. Fungi that inhabit the soil are also sensitive to contaminants (e.g., herbicides, insecticides, fungicides and heavy metals) that cause decreased or delayed growth, abnormalities of cellular metabolic pathways, and damage of cellular structures (Różalska et al. 2014; Gola et al. 2018; Szewczyk et al. 2018; Stolarek et al. 2019; Subbanna et al. 2019). Adverse changes in the cells caused by these compounds prevent these microorganisms from playing their proper ecological role. Some fungi have also developed mechanisms that allow them to survive in the presence of such contaminants (Szewczyk et al. 2018; Nowak et al. 2019).
Because of high enzymatic activity, the ability to produce secondary metabolites, and good growth in culture media, EPF are also being tested for use in other fields of biotechnology, e.g., nanoparticle biosynthesis and bioconversion of steroids or flavonoids, which are important from an economic point of view (Różalska et al. 2016; Gonzalez et al. 2017; Yosri et al. 2018; Dou et al. 2019; Kozłowska et al. 2019).
2 Entomopathogenic fungi—short description
Entomopathogenic fungi are parasitic microorganisms with an ability to infect and kill arthropods. They are mainly used as biopesticides in ecologic farming as a safe alternative to toxic chemical insecticides, although some of them are also used in biotechnological processes or in Chinese medicine (Jaihan et al. 2016; Ríos-Moreno et al. 2016; Lovett and Leger 2017).
Entomopathogenic fungi do not form one monophyletic group. Thus far, 12 species of Oomycetes, 65 species of Chytridiomycota, 339 species of Microsporidia, 474 species of Entomophtoromycota, 238 species of Basidiomycota, and 476 species of Ascomycota have been reported (Araújo and Hughes 2016). Species belonging to Ascomycota and Entomophtoromycota occur most frequently in nature. In the literature, species belonging to Ascomycota to the genera Metarhizium (M. anisopliae, M. robertsii, M. brunneum,M. lepidiotae, M. globosum, M. acridum, M. majus, M. flavoviride, M. rileyi, M. **shaense, M. lepidiotae and M. guizhouense), Beauveria (B. bassiana and B. brongniartii), Isaria (I. fumosorosea—formerly Paecilomyces fumosoroseus, I. farinosa and I. tennuipes), Ophiocordyceps (O. sinensis—formerly Cordyceps sinensis, O. unilateralis), Cordyceps (C. militaris), Torubiella (T. ratticaudata), Pochonia (P. chlamydosporia), Lecanicillium (L. lecani—formerly Verticillium lecanii, L. longisporum), Hirsutella (H. thompsonii, H. nodulosa, H. aphidis), and the species Paecilomyces variotii, Purpureocillium lilacinum are described (Khan et al. 2012; Tkaczuk et al. 2015; Jaihan et al. 2016.
Among the known EPF, Entomophthorales (e.g., Furia, Conidiobolus, Entomophaga, or Erynia) show the highest insecticidal activity; however, because of technical difficulties in breeding in laboratory conditions, they are not used as components of biopreparations. The most commonly used EPF are easily cultured saprotrophic fungi belonging to Ascomycota (Mascarin and Jaronski 2016). Worldwide, several different biopesticides based on species belonging to the genera Metarhizium, Beauveria, Paecilomyces, Isaria and Lecanicillium have been used. These fungi have a wide spectrum of activity, and they can therefore infect a wide variety of arthropod species (Khan et al. 2012; Castro et al. 2016; Ríos-Moreno et al. 2016).
Entomopathogenic fungi are heterogeneous organisms that play various ecological roles. For example, species of the genus Metarhizium and Beauveria, which are commonly found in soil, not only control natural arthropod populations but also form complex relationships with plants. They are described as endophytes of plant roots, stems, and leaves (Jaber and Enkerli 2017). It has been shown that M. robertsii and B. bassiana (but not L. lecani) provide plants with nitrogen that is assimilated during the parasitization of insects (Behie and Bidochka 2014), thus supporting plant growth (Ríos-Moreno et al. 2016). Beauveria bassiana acts as an endophyte in about 25 plant species, contributing to the control of pests and fungal plant pathogens (McKinnon et al. 2017; Vega 2018). As a fungal endophyte and epiphyte, it colonizes leaves and shoots in addition to plant roots making plants more resistant to insects (Klieber and Reineke 2016; Ramakuwela et al. 2020), and also successfully protects plants from microbial pathogens by suppressing disease-causing agents or increasing plant defense responses (Moonjely et al. 2016). Similarly, Lecanicillium also prevents fungal disease by growing on the surface of leaves, limiting available nutrients and producing antimicrobial compounds in addition to inducing plant responses while colonizing plant roots (Moonjely et al. 2016). Among EPF, I. fumosorosea seems not to fall into strong interactions with plants. This is probably due to the fact that Isaria spp. are sensitive to chemical substances secreted by plants and belonging to the defense system against plant pathogens (Lacey and Mercadier 1998; Zimmermann 2008). Interestingly, isolates of I. fumosorosea are reported to act against the root nematode, Meloidogyne javanica, although infection rates were found to be very low (below 40%) (Tigano-Milani et al. 1995). EPF are primarily used in biocontrol of insect pests in the laboratory, greenhouse or in the field.
2.1 The infectious process
Entomopathogenic fungi infect insects by direct penetration of the cuticle. Unlike bacteria or viruses, they do not have to be ingested by an insect (Bilgo et al. 2018). The infection process starts with the adhesion of spores to arthropod shells and has two stages: the first depends on the action of hydrophobic and electrostatic forces and the second requires the activity of enzymes and low-molecular-weight proteins called hydrophobins (Skinner et al. 2014). Spore germination occurs in the presence of carbon and energy sources on the insect’s cuticle at sufficient humidity and temperature. The optimum temperature for the growth and germination of EPF is between 20 and 30 °C. Spores can also germinate at temperatures outside this range and this is a characteristic feature of the particular fungal strain (Skinner et al. 2014). Subsequently, appressoria emerge, causing strong mechanical pressure on the cuticle and the production of lytic enzymes (proteo-, lipo- and chitinolytic) that disintegrate the insect’s body shells (Skinner et al. 2014; Lacey et al. 2015). After penetrating the arthropod’s body cavity (hemocel), the fungal hyphae start to grow. Some EPF can produce blastospores that enter host’s hemolymph and produce secondary hyphae that inhabit the host’s tissues. At this stage, the fungi produce secondary metabolites that cause paralysis and disrupt the host’s physiological processes, mainly its immune responses (Donzelli and Krasnoff 2016). Because of the develo** infection, the insect’s body is destroyed by both mechanical damage to the internal organs by the develo** hyphae and nutrient depletion (Donzelli and Krasnoff 2016; Mascarin and Jaronski 2016; Fan et al. 2017).
As a result of the progressive infection, the insect’s body, initially soft, becomes stiff due to fluid absorption by the fungus. Cadavers of insects attacked by fungi of the genus Beauveria may initially take on a dark red color. The entire infection process is relatively long and takes approximately 14 days after infection, but first symptoms of infection usually occur about 7 days post infection (or even earlier, depending on fungal species). After killing the insect and using all nutrition, the hyphae of the fungus emerge from the cadaver of the host through holes in its body (mouth hole, anus) and through intersegmental areas. Then, resting or infective spores are produced, which allows the fungus to spread and infect other individuals (Skinner et al. 2014).
2.2 Infectious agents
The pathogenesis caused by EPF requires the involvement of several infectious agents, the most important of which are adhesins, lytic enzymes, and secondary metabolites.
2.2.1 Adhesins
The first stage of the fungal infection process is spore adhesion to the surface of the arthropod’s body. In this stage, two types of proteins are produced: hydrophobins (whose layers disintegrate during the sporulation of spores) and adhesins (MAD1 and MAD2), which enable both close adhesion to the insect’s cuticle and recognition of the host by the fungal pathogen (Wang and Leger 2007; Greenfield et al. 2014).
2.2.2 Lytic enzymes
Lytic enzymes play the most important role during the process of insect infection by EPF. Their action, structure, and types are relatively well described. Their main role is hydrolysis of the components of the insect cuticle, which allows appressoria to penetrate the outer covers of arthropods. Lytic enzymes are produced as soon as the spore attaches to the cuticle and begins to form appressorium (Santi et al. 2010).
Lipases are produced first and hydrolyze the lipids and lipoproteins located in the insect epicuticle (outer cuticle) (Pedrini et al. 2007). These enzymes hydrolyze the ester bonds of triacylglycerols, which leads to the release of free fatty acids, diacylglycerols, monoacylglycerols, and glycerol (Silva et al. 2009). Moreover, lipases support the adhesion of germinating spores to insect cuticles by increasing hydrophobic interactions between the fungus and the cuticle surface (Santi et al. 2010). All EPF have been shown to contain these enzymes, most of which have been well described (Supakdamrongkul et al. 2010; Mondal et al. 2016). One of these enzymes is M. brunneum (formerly M. anisopliae var. anisopliae) phospholipase C, which hydrolyzes phosphodiester bonds and is responsible for degrading phospholipids of insect cell membranes, which allows the fungus to enter the insect’s hemocel and infect its tissues (Santi et al. 2010).
A key factor in the virulence of EPF is proteolytic enzymes that hydrolyze peptide bonds of insect cuticles, leading to the expsore of chitin fibrils. One of the first comprehensively described proteases is subtilisin (Pr1, serine endoprotease), which degrades both cuticle proteins and modifies the surface of the cuticle, thereby facilitating spore adhesion. This endoprotease is present in M. anisopliae (Shah et al. 2005), O. sinensis (Zhang et al. 2008), and B. bassiana (Donatti et al. 2008). However, other proteases such as trypsin-like acid Pr2 protease, cysteine Pr4 proteinase, and metalloprotease in M. anisopliae; Pr1- and Pr2-like serine proteases in B. brongniartii, L. lecanii, M. rileyi, and Aschersonia aleyrodis; and serine elastase in Conidiobolus coronatus and B. bassiana also participate in the pathogenesis process (Zhao et al. 2016). Some studies indicate that proteases of EPF play a dual role in the infectious process. Although their main function is hydrolysis of cuticular proteins, proteases can also participate in the inactivation of antifungal proteins produced in the epidermis of insects (Sotelo-Mundo et al. 2007).
The last group of enzymes are chitinases that are classified as follows depending on the place of their action on the chitin molecule: endochitinases (hydrolyze the β-1,4-glycosidic bond inside the chitin molecule) and exochitinases (hydrolyze N-acetylglucosamine oligomers formed during the action of endochitinases). The combined action of endo- and exochitinases is required for the complete degradation of insect chitin. Chitinolytic enzymes have been found in numerous EPF (Duo-Chuan 2006).
Apart from proteases, lipases, and chitinases, EPF also produce other enzymes that are not directly involved in the digestion of the cuticle but play an important role in pathogenesis. One of them is acid trehalase (ATM1) that hydrolyzes trehalose, the main disaccharide of insect hemolymph, with the release of two glucose molecules, thus providing a nutrient for EPF. Inactivation of the ATM1 gene in M. acridum causes a significant reduction in the virulence of this strain, which is manifested as the inability of this species to grow inside the insect’s body (** et al. 2019) determined the effect of five heavy metals (Zn (II), Ni (II), Cu (II), Cd (II), and Pb (II)) on the growth of I. farinosa, I. fumosorosea, and I. tennuipes. The experiments were conducted on Sabouraud solid medium supplemented with heavy metals at concentrations that normally occur in soil (i.e., Zn (II): 33.0; Pb(II): 13.8; Cu (II), Ni (II): 6.5; Cd (II): 0.22 mg L−1) and at concentrations that were 10 and 100 times higher. The obtained data revealed that the greatest toxic effect of heavy metal ions on the EPF was observed when their concentration was 100 times higher than the natural content (on average, approximately 50.5% inhibition of growth). The greatest inhibitory effect on the growth of the fungal colonies was showed by Ni (II), while Pb (II) showed the least effects. Among all the tested species, I. fumosorosea had the highest tolerance to the presence of heavy metals.
Plants are widely used for bioremediation of heavy metal-contaminated areas. They interact closely with microorganisms, which can enhance plant growth and health by increasing nutrient uptake and improving plant resistance to pathogens and stress (Kidd et al. 2017). EPF are not only insect pathogens, but they are also associated with plant rhizosphere, thereby promoting plant growth. Farias et al. (2019) investigated the effect of fungal consortium containing four species of EPF (B. bassiana, M. anisopliae, P. chlamydosporia, and P. lilacinum) on the ability of Jacaranda mimosifolia to absorb Cu (II), Zn (II), and Mn (II). After 45 days of experiments, it was found that the use of the fungal preparation in combination with biochar caused an increase in the mass of roots and shoots of J. mimosifolia, intensified the translocation of metals from roots to shoots, and inhibited the leaching of metals from plant tissues, which resulted in an increased concentration of these metals in J. mimosifolia tissues. The application of the consortium of fungal isolates and biochar improved the phytoremediation potential of J. mimosifolia.
6 Nanoparticle biosynthesis by entomopathogenic fungi
During the last decade, metal nanoparticles have received considerable attention due to their wide range of applications in many different fields (e.g., pest management). They have also been used as antimicrobial agents and biomarkers. The production of nanoparticles by fungi is an interesting and ecological alternative to chemical and physical syntheses. Extracts from EPF or their culture media containing numerous enzymes can also serve as catalysts for the production of nanoparticles.
The waste mycelium after biodegradation processes is a valuable source of both enzymes and many other substances that can be reused in various processes. An example is the use of M. robertsii waste biomass (obtained after nonylphenol degradation) for the production of silver nanoparticles (AgNPs). Aqueous extracts from the waste mycelium M. robertsii, in dilutions of 25% and 50%, allowed the synthesis of silver nanoparticles from silver nitrate, and the obtained nanoparticles were small in size (from 2 to 29 nm) and relatively monodisperse, which is an important factor in their later use. The obtained colloids also exhibited antimicrobial activity, and the addition of 30 ppm AgNPs caused growth inhibition of S. aureus and E. coli (Różalska et al. 2016). These nanoparticles were also found to have fungicidal properties against Candida albicans, C. glabrata, and C. parapsilosis minimum inhibitory concentration (MIC) values ranged from 1.56 to 6.25 µg mL−1. An 85% decrease in viability of the tested yeast was observed after 6 h of incubation with AgNPs (added at a concentration of 6.25 µg mL−1); it was also observed that this concentration of nanoparticles was not toxic to mammalian cells. The main mechanism of action of AgNPs against pathogenic yeast is probably based on changes in membrane permeability associated with a decrease in the amount of C18:2 phospholipids (Różalska et al. 2018).
In develo** countries, the major public health concerns are vector-borne diseases. EPF are frequently used in protection against mosquitoes. Moreover, nanoparticles produced by these microorganisms were also found to be effective in biocontrol programs.
Myco-synthesized AgNPs are also a potential larvicidal and eco-friendly agent for controlling mosquito population. AgNPs obtained from M. anisopliae culture extracts may be relevant as biocontrol agents for the malaria vector Anopheles culicifacies mosquitos. The LC50 (median lethal concentration) of the studied nanoparticles ranged from 32 to 51 mg L−1 for various larval stages and 60 mg L−1 for pupae, although the low doses of silver nanoparticles limited the development of larvae and pupae (Amerasan et al. 2016). AgNPs synthesized by I. fumosorosea are even more effective against mosquitos. These nanoparticles were found to be more susceptible against all larval instars of Culex quinquefasciatus and Aedes aegypti. The mortality of all larval instars in the presence of 1 ppm of AgNPs ranged from 92% to 100%. The LC50 and LC90 values were 0.240–0.652 and 1.219–2.916, respectively, for all larval instars (I–IV) of C. quinquefasciatus and 0.065–0.137 and 0.558–1.278 ppm for A. aegypti (Banu and Balasubramanian 2014). The filtrates of I. fumosorosea can also produce iron nanoparticles that can serve as an insecticide against sweet potato whitefly Bemisia tabaci. These nanoparticles showed high pathogenicity against nymphs (second and third instar) and pupae, with LC50 values ranging from 19.17 to 37.71 ppm. LT50 (lethal time) at the iron nanoparticle concentration of 50 ppm was shorter for second instar nymphs (3.15 days) and longer for pupae (4.22 days) (Wang et al. 2019a).
Metarhizium anisopliae-synthesized titanium nanoparticles (TiNPs) also have insecticidal potential. The larvicidal activity of TiNPs against G. mellonella was 22%, but the synergistic effect of these nanoparticles and gamma-irradiated M. anisopliae (with higher lipase, protease, amylase, and nitrate reductase activities) caused 82% larval mortality (Yosri et al. 2018).
7 Biotransformation performed using entomopathogenic fungi
Entomopathogenic fungi can perform biotransformation, leading to the generation of derivatives with higher activity. These processes are favorable, because they improve the absorbability of products (Yang et al. 2018; Dou et al. 2019; Kozłowska et al. 2019).
7.1 Steroid biotransformation
Steroids are compounds with high economic importance, and they are widely used in medicine as hormones and active drugs. Compounds of pharmaceutical significance are obtained by modification and transformation. Because chemical methods of steroid modification are very complicated and involve even several stages, transformations are most often performed using microorganisms. Microbiological transformations of steroids include hydroxylation, Baeyer–Villiger oxidation, reduction, and isomerization. Among them, 11α-, 11β-, and 17α-hydroxylation reactions carried out by filamentous fungi are the key reactions to obtain molecules with high biological activity (**ong et al. 2006; Gonzalez et al. 2017; Kozłowska et al. 2019).
Many filamentous fungi are used in steroid transformations, and EPF can also be efficiently used for transformations. Recent studies have shown that 12 I. farinosa strains could effectively transform dehydroepiandrosterone (DHEA) to 7α- and 7β-hydroxy derivatives. One of these strains, I. farinosa KCh KW1.1, transformed androstenediol, androstenedione, adrenosterone, 17α-methyltestosterone, 17β-hydroxyandrost-1,4,6-triene-3-one, and progesterone to mono or dihydroxy derivatives with a high yield and stereoselectivity (Kozlowska et al. 2019).
In another study, biotransformation of estrogens (E1, E2, and EE2) by I. fumosorosea was confirmed. Most of the estrogens were hydroxylated at C-6 position and glycosylated at C-3 position. The presence of a methyl group at the C-3 steroid ring completely inhibited the glycosylation of estrogens by I. fumosorosea, indicating that a free hydroxyl group at this position is necessary for glycosylation (Kozłowska et al. 2019).
Entomopathogenic fungi can also transform steroids on a large scale, which can be used for industrial purpose in the future. 11α-Hydroxy was transformed to 13-ethyl-gon-4-ene-3,17-dione (GD, a key step in the synthesis of oral contraceptive desogestrel) by M. anisopliae and nano-liposome technique with a high yield. The main reaction product 11α-hydroxy-13-ethyl-gon-4-ene-3,17-dione (HGD) was also produced at high concentration of GD as 6 g L−1 with no reduction of transformation efficiency. The main byproduct of the reaction 6β,11α-dihydroxy derivative was found to benefit the purification of HGD (Feng et al. 2014).
Beauveria bassiana may also find applications in the pharmaceutical industry because it gives high yields of valuable 11α-hydroxy intermediates of steroids (Gonzalez et al. 2017). This process, however, seems to require optimization. In B. bassiana culture media with androst-4-ene-3,17-dione at pH = 6.0, only two hydroxylated metabolites were found, while in samples at pH = 7.0, four hydroxylated and/or reduced metabolites were detected. These data show that B. bassiana is clearly an efficient biocatalyst for 11α-hydroxylation and reduction of the 17-carbonyl group (**ong et al. 2006). B. bassiana strain ATCC7159 transforms DHEA to 3β,11α,17β-trihydroxyandrost-5-ene in the presence of a moderate DHEA concentration (0.83–1.0 g L−1) and in a mild environment (26 °C, pH 7) (Gonzalez et al. 2017). The addition of various compounds can positively affect the biosynthesis of these pharmaceutical metabolites. An example is the enhanced hydroxylation capacity of B. bassiana with n-alkane solvents as a carbon source. The addition of n-dodecane (n-C12) and n-hexadecane (n-C16) was found to enhance the conversion of DHEA to 3β,11α,17β-trihydroxyandrost-5-ene, resulting in the appearance 22.8% mM hydroxylated product in the culture medium. An unusual feature of Beauveria is the mode of conducting the reaction, which consists of the reduction of the C-17 ketone of DHEA preceded by 11α-hydroxylation. This phenomenon differs from the reaction carried out by other microorganisms, which first activate an unfunctionalized carbon to 11α-hydroxy-17-oxo derivative before obtaining a 3β,11α,17β-triol product (Gonzalez et al. 2015).
7.2 Flavonoid biotransformation
Flavonoids are derivatives of plant polyphenols. These are glycosides with O- and C-glycosidic bonds connecting the base with sugar residues (Dymarska et al. 2018a, b; Dou et al. 2019), including most often glucose, rhamnose, galactose, xylose, and arabinose (Yang et al. 2018). It is believed that the sugar group of flavonoids is the main factor affecting the absorption of these compounds by the human body (Sordon et al. 2017). There are six classes of flavonoids: flavones, isoflavones, flavanones, flavanols, flavanonols, and flavan-3-ols. Because of their antioxidant, anti-inflammatory, and antibacterial properties, they exert beneficial effects on human health. In addition, flavonoids have anti-tumor and immunomodulatory activity and protective effects on the human nervous system. The consumption of foods containing flavonoids can prevent the development of heart disease, diabetes, and depression (Dymarska et al. 2018a; Yang et al. 2018; Dou et al. 2019). They occur naturally in vegetables, fruits, cereals, wine, and tea (Yang et al. 2018; Dou et al. 2019).
Microorganisms can efficiently biotransform flavonoids to flavonoid glycosides, which are better absorbed than flavonoids themselves. Among microorganisms, EPF seem to have great potential in these processes; however, currently, Isaria and Beauveria are studied frequently, and the potential of Metarhizium remains undiscovered. The relevant data are presented in Table 1.
8 Conclusions
A review of literature showed that in addition to the use of EPF in biocontrol, these fungi can also be used in different ways that have not yet been described in previous reviews. EPF can also be used for removing harmful substances from the environment, because they possess enzymes that enable the removal of toxic compounds of anthropogenic origin. For example, M. robertsii and M. brunneum species can degrade nonylphenols, triazines, tin compounds, synthetic estrogen, hydrocarbons, and even industrial dyes. These microorganisms also interact with heavy metals, which sometimes adversely affect their growth or hyphae morphology; however, these fungi possess the ability to remove heavy metals from the environment, for example, from contaminated waters (zinc, copper, cadmium, nickel, and chromium) and indirectly by improving the phytoremediation properties of plants (copper, manganese, and zinc). On the basis of this current review article, the authors conclude that EPF have great potential in the biosynthesis of biologically active compounds used in medicine, for example, hydroxylated steroids and glycated flavonoids or for bioproduction of silver nanoparticles with antibacterial and antifungal properties. Because of the high potential shown by EPF and the many different functions that they can perform, they are being increasingly researched.
References
Acir IH, Guenther K (2018) Endocrine-disrupting metabolites of alkylphenol ethoxylates—a critical review of analytical methods, environmental occurrences, toxicity, and regulation. Sci Total Environ 635:1530–1546. https://doi.org/10.1016/j.scitotenv.2018.04.079
Ali S, Huang Z, Ren S (2013) Effect of fungicides on growth, germination and cuticle-degrading enzyme production by Lecanicillium muscarium. Biocontrol Sci Technol 23:711–723. https://doi.org/10.1080/09583157.2013.794258
Amerasan D, Nataraj T, Murugan K et al (2016) Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae). J Pest Sci 89:249–256. https://doi.org/10.1007/s10340-015-0675-x
Amutha M, Gulsar Banu J, Surulivelu T, Gopalakrishnan N (2010) Effect of commonly used insecticides on the growth of white Muscardine fungus, Beauveria bassiana under laboratory conditions. J Biopestic 3:143–146
Araújo JPM, Hughes DP (2016) Diversity of Entomopathogenic Fungi Which groups conquered the insect body? In: Lovett B, Leger RJS (eds) Advances in genetics, vol 94. Elsevier, Amsterdam, pp 1–39. https://doi.org/10.1016/bs.adgen.2016.01.001
Arroyo-Manzanares N, Diana Di Mavungu J, Garrido-Jurado I et al (2017) Analytical strategy for determination of known and unknown destruxins using hybrid quadrupole-Orbitrap high-resolution mass spectrometry. Anal Bioanal Chem 409:3347–3357. https://doi.org/10.1007/s00216-017-0276-z
Ashraf S, Radhi M, Gowler P et al (2019) The polyadenylation inhibitor cordycepin reduces pain, inflammation and joint pathology in rodent models of osteoarthritis. Sci Rep 9:4696. https://doi.org/10.1038/s41598-019-41140-1
Asi MR, Bashir MH, Afzal M et al (2010) Compatibility of entomopathogenic fungi, Metarhizium anisopliae and Paecilomyces fumosoroseus with selective insecticides. Pak J Bot 42:4207–4214
Avery PB, Pick DA, Aristizábal LF et al (2013) Compatibility of Isaria fumosorosea (Hypocreales: Cordycipitaceae) blastospores with agricultural chemicals used for management of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects 4:694–711. https://doi.org/10.3390/insects4040694
Bagga S, Hu G, Screen SE, Leger RJS (2004) Reconstructing the diversification of subtilisins in the pathogenic fungus Metarhizium anisopliae. Gene 324:159–169. https://doi.org/10.1016/j.gene.2003.09.031
Banu AN, Balasubramanian C (2014) Optimization and synthesis of silver nanoparticles using Isaria fumosorosea against human vector mosquitoes. Parasitol Res 113:3843–3851. https://doi.org/10.1007/s00436-014-4052-0
Behie SW, Bidochka MJ (2014) Ubiquity of insect-derived nitrogen transfer to plants by endophytic insect-pathogenic fungi: an additional branch of the soil nitrogen cycle. Appl Environ Microbiol 80:1553–1560. https://doi.org/10.1128/AEM.03338-13
Bilgo E, Lovett B, Leger RJS et al (2018) Native entomopathogenic Metarhizium spp. from Burkina Faso and their virulence against the malaria vector Anopheles coluzzii and non-target insects. Parasites Vectors 11:11–16. https://doi.org/10.1186/s13071-018-2796-6
Bohnert M, Dahse HM, Gibson DM et al (2013) The fusarin analog NG-391 impairs nucleic acid formation in K-562 leukemia cells. Phytochem Lett 6:189–192. https://doi.org/10.1016/J.PHYTOL.2013.01.001
Carpio A, Arroyo-Manzanares N, Ríos-Moreno A et al (2016) Development of a QuEChERS-based extraction method for the determination of destruxins in potato plants by UHPLC–MS/MS. Talanta 146:815–822. https://doi.org/10.1016/j.talanta.2015.06.008
Castro T, Mayerhofer J, Enkerli J et al (2016) Persistence of Brazilian isolates of the entomopathogenic fungi Metarhizium anisopliae and M. robertsii in strawberry crop soil after soil drench application. Agric Ecosyst Environ 233:361–369. https://doi.org/10.1016/j.agee.2016.09.031
Celar FA, Kos K (2016) Effects of selected herbicides and fungicides on growth, sporulation and conidial germination of entomopathogenic fungus Beauveria bassiana. Pest Manag Sci 72:2110–2117. https://doi.org/10.1002/ps.4240
D’Alessandro CP, Padin S, Urrutia MI, López Lastra CC (2011) Interaction of fungicides with the entomopathogenic fungus Isaria fumosorosea. Biocontrol Sci Technol 21:189–197. https://doi.org/10.1080/09583157.2010.536200
Dara SK (2017) Compatibility of the entomopathogenic fungus Beauveria bassiana with some fungicides used in California strawberry. Open Plant Sci J 10:29–34
Demirci F, Muştu M, Kaydan MB, Ülgentürk S (2011) Effects of some fungicides on Isaria farinosa, and in vitro growth and infection rate on Planococcus citri. Phytoparasitica 39:353–360. https://doi.org/10.1007/s12600-011-0168-2
Donatti AC, Furlaneto-Maia L, Fungaro MHP, Furlaneto MC (2008) Production and regulation of cuticle-degrading proteases from Beauveria bassiana in the presence of Rhammatocerus schistocercoides cuticle. Curr Microbiol 56:256–260. https://doi.org/10.1007/s00284-007-9071-y
Donzelli BGG, Krasnoff SB (2016) Molecular genetics of secondary chemistry in Metarhizium Fungi. In: Lovett B, Leger RJS (eds) Advances in genetics, vol 94. Elsevier, Amsterdam, pp 365–436
Donzelli BGG, Krasnoff SB, Churchill ACL et al (2010) Identification of a hybrid PKS–NRPS required for the biosynthesis of NG-391 in Metarhizium robertsii. Curr Genet 56:151–162. https://doi.org/10.1007/s00294-010-0288-0
Dou F, Wang Z, Li G, Dun B (2019) Microbial transformation of flavonoids by Isaria fumosorosea ACCC 37814. Molecules 24:1028. https://doi.org/10.3390/molecules24061028
Duo-Chuan L (2006) Review of fungal chitinases. Mycopathologia 161:345–360. https://doi.org/10.1007/s11046-006-0024-y
Dymarska M, Janeczko T, Kostrzewa-Susłow E (2018a) Biotransformations of flavones and an isoflavone (daidzein) in cultures of entomopathogenic filamentous fungi. Molecules 23(6):1356. https://doi.org/10.3390/molecules23061356
Dymarska M, Janeczko T, Kostrzewa-Susłow E (2018b) Glycosylation of 3-Hydroxyflavone, 3-Methoxyflavone, quercetin and baicalein in fungal cultures of the Genus Isaria. Molecules 23(10):pii: E2477. https://doi.org/10.3390/molecules23102477
Fan Y, Liu X, Keyhani NO et al (2017) Regulatory cascade and biological activity of Beauveria bassiana oosporein that limits bacterial growth after host death. Proc Natl Acad Sci USA 114:E1578–E1586. https://doi.org/10.1073/pnas.1616543114
Farias CP, Alves GS, Oliveira DC et al (2019) A consortium of fungal isolates and biochar improved the phytoremediation potential of Jacaranda mimosifolia D. Don and reduced copper, manganese, and zinc leaching. J Soils Sediments. https://doi.org/10.1007/s11368-019-02414-3
Feng M, Liao Z, Han L et al (2014) Enhancement of microbial hydroxylation of 13-ethyl-gon-4-ene-3,17-dione by Metarhizium anisopliae using nano-liposome technique. J Ind Microbiol Biotechnol 41:619–627. https://doi.org/10.1007/s10295-014-1414-7
Feng P, Shang Y, Cen K, Wang C (2015) Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity. Proc Natl Acad Sci 112:11365–11370. https://doi.org/10.1073/pnas.1503200112
Fiedler Z, Sosnowska D (2017) Side effects of fungicides and insecticides on entomopathogenic fungi in vitro. J Plant Prot Res 57:355–360. https://doi.org/10.1515/jppr-2017-0048
Forlani L, Juárez MP, Lavarías S, Pedrini N (2014) Toxicological and biochemical response of the entomopathogenic fungus Beauveria bassiana after exposure to deltamethrin. Pest Manag Sci 70:751–756. https://doi.org/10.1002/ps.3583
Gola D, Dey P, Bhattacharya A et al (2016) Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresour Technol 218:388–396. https://doi.org/10.1016/j.biortech.2016.06.096
Gola D, Malik A, Namburath M, Ahammad SZ (2018) Removal of industrial dyes and heavy metals by Beauveria bassiana: FTIR, SEM, TEM and AFM investigations with Pb(II). Environ Sci Pollut Res 25:20486–20496. https://doi.org/10.1007/s11356-017-0246-1
Gonzalez R, Nicolau F, Peeples T (2015) N-alkane Solvent-enhanced Biotransformation of Steroid DHEA by Beauveria bassiana as Biocatalyst. J Adv Biol Biotechnol 2:30–37. https://doi.org/10.9734/jabb/2015/13541
Gonzalez R, Nicolau F, Peeples TL (2017) Optimization of the 11α-hydroxylation of steroid DHEA by solvent-adapted Beauveria bassiana. Biocatal Biotransformation 35:103–109. https://doi.org/10.1080/10242422.2017.1289183
Greenfield BPJ, Lord AM, Dudley E, Butt TM (2014) Conidia of the insect pathogenic fungus, Metarhizium anisopliae, fail to adhere to mosquito larval cuticle. R Soc Open Sci 1(2):140193. https://doi.org/10.1098/rsos.140193
Haruma T, Yamaji K, Ogawa K et al (2019) Root-endophytic Chaetomium cupreum chemically enhances aluminium tolerance in Miscanthus sinensis via increasing the aluminium detoxicants, chlorogenic acid and oosporein. PLoS ONE 14:e0212644. https://doi.org/10.1371/journal.pone.0212644
Heilos D, Röhrl C, Pirker C et al (2018) Altered membrane rigidity via enhanced endogenous cholesterol synthesis drives cancer cell resistance to destruxins. Oncotarget 9:25661–25680. https://doi.org/10.18632/oncotarget.25432
Hirose E, Neves PMOJ, Zequi JAC et al (2001) Effect of biofertilizers and neem oil on the entomopathogenic fungi Beauveria bassiana (bals.) vuill. and Metarhizium anisopliae (Metsch.) sorok. Brazilian Arch Biol Technol 44:419–423. https://doi.org/10.1590/S1516-89132001000400013
Huarte-Bonnet C, Juárez MP, Pedrini N (2015) Oxidative stress in entomopathogenic fungi grown on insect-like hydrocarbons. Curr Genet 61:289–297. https://doi.org/10.1007/s00294-014-0452-z
Huarte-Bonnet C, Kumar S, Saparrat MCN et al (2018) Insights into Hydrocarbon Assimilation by Eurotialean and Hypocrealean Fungi: roles for CYP52 and CYP53 Clans of Cytochrome P450 Genes. Appl Biochem Biotechnol 184:1047–1060. https://doi.org/10.1007/s12010-017-2608-z
Hussein K, Hassan S, Joo J (2011) Potential capacity of Beauveria bassiana and Metarhizium anisopliae in the biosorption of Cd2+ and Pb2+. J Gen Appl Microbiol 57(6):347–355
Jaber LR, Enkerli J (2017) Fungal entomopathogens as endophytes: can they promote plant growth? Biocontrol Sci Technol 27:28–41. https://doi.org/10.1080/09583157.2016.1243227
Jaihan P, Sangdee K, Sangdee A (2016) Selection of entomopathogenic fungus for biological control of chili anthracnose disease caused by Colletotrichum spp. Eur J Plant Pathol 146:551–564. https://doi.org/10.1007/s10658-016-0941-7
Jajić I, Dudaš T, Krstović S et al (2019) Emerging fusarium mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin in serbian maize. Toxins (Basel) 11:357. https://doi.org/10.3390/toxins11060357
** K, Peng G, Liu Y, **a Y (2015) The acid trehalase, ATM1, contributes to the in vivo growth and virulence of the entomopathogenic fungus, Metarhizium acridum. Fungal Genet Biol 77:61–67. https://doi.org/10.1016/j.fgb.2015.03.013
Keswani C, Singh HB, Hermosa R et al (2019) Antimicrobial secondary metabolites from agriculturally important fungi as next biocontrol agents. Appl Microbiol Biotechnol 103:9287–9303
Keyhani NO (2018) Lipid biology in fungal stress and virulence: entomopathogenic fungi. Fungal Biol 122:420–429. https://doi.org/10.1016/j.funbio.2017.07.003
Khan S, Guo L, Maimaiti Y et al (2012) Entomopathogenic fungi as microbial biocontrol agent. Mol Plant Breed. https://doi.org/10.5376/mpb.2012.03.0007
Kidd PS, Alvarez-Lopez V, Becerra-Castro C et al (2017) Chapter three—potential role of plant-associated bacteria in plant metal uptake and implications in phytotechnologies. Adv Bot Res 83:87–126
Klieber J, Reineke A (2016) The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J Appl Entomol 140:580–589. https://doi.org/10.1111/jen.12287
Kouassi M, Coderre D, Todorova SI (2003) Effects of the timing of applications on the incompatibility of three fungicides and one isolate of the entomopathogenic fungus Beauveria bassiana (Balsamo) Vuillemin (Deuteromycotina). J Appl Entomol 127:421–426
Kozłowska E, Dymarska M, Kostrzewa-Susłow E, Janeczko T (2019) Cascade biotransformation of estrogens by Isaria fumosorosea KCh J2. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-47225-1
Kumar R, Kumawat N, Sahu YK (2017) Role of biofertilizers in agriculture. Pop Kheti 5:63–66
Lacey LA, Mercadier G (1998) The effect of selected allelochemicals on germination of conidia and blastospores and mycelial growth of the entomopathogenic fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes). Mycopathologia 142:17–25. https://doi.org/10.1023/A:1006963016316
Lacey LA, Grzywacz D, Shapiro-Ilan DI et al (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41. https://doi.org/10.1016/j.jip.2015.07.009
Latteur G, Jansen JP (2002) Effects of 20 fungicides on the infectivity of conidia of the aphid entomopathogenic fungus Erynia neoaphidis. Biocontrol 47:435–444. https://doi.org/10.1023/A:1015639017666
Liu BL, Tzeng YM (2012) Development and applications of destruxins: a review. Biotechnol Adv 30:1242–1254. https://doi.org/10.1016/j.biotechadv.2011.10.006
Lovett B, Leger RJS (2017) The insect pathogens. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.funk-0001-2016
Mascarin GM, Jaronski ST (2016) The production and uses of Beauveria bassiana as a microbial insecticide. World J Microbiol Biotechnol 32:1–26. https://doi.org/10.1007/s11274-016-2131-3
Mc Namara L, Dolan SK, Walsh JMD et al (2019) Oosporein, an abundant metabolite in Beauveria caledonica, with a feedback induction mechanism and a role in insect virulence. Fungal Biol 123:601–610. https://doi.org/10.1016/j.funbio.2019.01.004
McKinnon AC, Saari S, Moran-Diez ME et al (2017) Beauveria bassiana as an endophyte: a critical review on associated methodology and biocontrol potential. Biocontrol 62:1–17. https://doi.org/10.1007/s10526-016-9769-5
Meyling NV, Arthur S, Pedersen KE et al (2018) Implications of sequence and timing of exposure for synergy between the pyrethroid insecticide alpha-cypermethrin and the entomopathogenic fungus Beauveria bassiana. Pest Manag Sci 74:2488–2495. https://doi.org/10.1002/ps.4926
Mondal S, Baksi S, Koris A, Vatai G (2016) Journey of enzymes in entomopathogenic fungi. Pacific Sci Rev A Nat Sci Eng 18:85–99. https://doi.org/10.1016/j.psra.2016.10.001
Moonjely SS, Barelli L, Bidochka MJ (2016) Insect pathogenic fungi as endophytes. In: Lovett B, Leger RJS (eds) Advances in genetics. Elsevier, Amsterdam, pp 107–135
Neves PMOJ, Hirose E, Tchujo PT, Moino A (2001) Compatibility of entomopathogenic fungi with neonicotinoid insecticides. Neotrop Entomol 30:263–268. https://doi.org/10.1590/s1519-566x2001000200009
Nowak M, Soboń A, Litwin A, Różalska S (2019) 4-n-nonylphenol degradation by the genus Metarhizium with cytochrome P450 involvement. Chemosphere 220:324–334. https://doi.org/10.1016/j.chemosphere.2018.12.114
Olleik H, Nicoletti C, Lafond M et al (2019) Comparative structure–activity analysis of the antimicrobial activity, cytotoxicity, and mechanism of action of the fungal cyclohexadepsipeptides enniatins and beauvericin. Toxins (Basel) 11:514. https://doi.org/10.3390/toxins11090514
Pedrini N, Rosana C, Patricia Juarez M (2007) Biochemistry of insect epicuticule degradation by entomopathogenic fungi. Comp Biochem Physiol 146:124–137
Ramakuwela T, Hatting J, Bock C et al (2020) Establishment of Beauveria bassiana as a fungal endophyte in pecan (Carya illinoinensis) seedlings and its virulence against pecan insect pests. Biol Control 140:104102. https://doi.org/10.1016/j.biocontrol.2019.104102
Ramesha A, Venkataramana M, Nirmaladevi D et al (2015) Cytotoxic effects of oosporein isolated from endophytic fungus Cochliobolus kusanoi. Front Microbiol 6:870. https://doi.org/10.3389/fmicb.2015.00870
Reddy DS, Reddy M, Pushpalatha M (2018) Interaction of fungicides with bio-control agents. J Entomol Zool Stud. 3:2098–2104
Ríos-Moreno A, Garrido-Jurado I, Resquín-Romero G et al (2016) Destruxin A production by Metarhizium brunneum strains during transient endophytic colonisation of Solanum tuberosum. Biocontrol Sci Technol 26:1574–1585. https://doi.org/10.1080/09583157.2016.1223274
Różalska S, Pawłowska J, Wrzosek M et al (2013) Utilization of 4-n-nonylphenol by Metarhizium sp. isolates. Acta Biochim Pol 60:677–682
Różalska S, Glińska S, Długoński J (2014) Metarhizium robertsii morphological flexibility during nonylphenol removal. Int Biodeterior Biodegrad 95:285–293. https://doi.org/10.1016/j.ibiod.2014.08.002
Różalska S, Bernat P, Michnicki P, Długoński J (2015) Fungal transformation of 17α-ethinylestradiol in the presence ofvarious concentrations of sodium chloride. Int Biodeterior Biodegrad 103:77–84. https://doi.org/10.1016/j.ibiod.2015.04.016
Różalska S, Soliwoda K, Długoński J (2016) Synthesis of silver nanoparticles from Metarhizium robertsii waste biomass extract after nonylphenol degradation, and their antimicrobial and catalytic potential. RSC Adv 6:21475–21485. https://doi.org/10.1039/c5ra24335a
Różalska B, Sadowska B, Budzyńska A et al (2018) Biogenic nanosilver synthesized in Metarhizium robertsii waste mycelium extract—as a modulator of Candida albicans morphogenesis, membrane lipidome and biofilm. PLoS ONE 13:1–21. https://doi.org/10.1371/journal.pone.0194254
Santi L, Beys da Silva WO, Berger M et al (2010) Conidial surface proteins of Metarhizium anisopliae: source of activities related with toxic effects, host penetration and pathogenesis. Toxicon 55:874–880. https://doi.org/10.1016/j.toxicon.2009.12.012
Shah FA, Wang CS, Butt TM (2005) Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol Lett 251:259–266. https://doi.org/10.1016/j.femsle.2005.08.010
Shah FA, Ansari MA, Watkins J et al (2009) Influence of commercial fungicides on the germination, growth and virulence of four species of entomopathogenic fungi. Biocontrol Sci Technol 19:743–753. https://doi.org/10.1080/09583150903100807
Siewiera P, Różalska S, Bernat P (2017a) Efficient dibutyltin (DBT) elimination by the microscopic fungus Metarhizium robertsii under conditions of intensive aeration and ascorbic acid supplementation. Environ Sci Pollut Res 24:12118–12127. https://doi.org/10.1007/s11356-017-8764-4
Siewiera P, Różalska S, Bernat P (2017b) Estrogen-mediated protection of the organotin-degrading strain Metarhizium robertsii against oxidative stress promoted by monobutyltin. Chemosphere 185:96–104. https://doi.org/10.1016/j.chemosphere.2017.06.130
Silva WOB, Santi L, Berger M et al (2009) Characterization of a spore surface lipase from the biocontrol agent Metarhizium anisopliae. Process Biochem 44:829–834. https://doi.org/10.1016/j.procbio.2009.03.019
Sivakumar T, Jiji T, Naseema A (2019) Effect of pesticides used in banana agro-system on entomopathogenic fungus, Metarhizium majus Bisch, Rehner and Humber. Int J Trop Insect Sci. https://doi.org/10.1007/s42690-019-00080-z
Skinner M, Parker BL, Kim JS (2014) Role of entomopathogenic fungi. In: Abrol DP (ed) Integrated pest management. Academic Press, Cambridge, pp 169–191
Sohrabi F, Jamali F, Morammazi S et al (2019) Evaluation of the compatibility of entomopathogenic fungi and two botanical insecticides tondexir and palizin for controlling Galleria mellonella L. (Lepidoptera: Pyralidae). Crop Prot 117:20–25
Sordon S, Popłónski J, Tronina T, Huszcza E (2017) Microbial glycosylation of daidzein, genistein and biochanin a: two new glucosides of biochanin A. Molecules 22(1):pii: E81. https://doi.org/10.3390/molecules22010081
Sotelo-Mundo RR, Lopez-Zavala AA, Garcia-Orozco KD et al (2007) The lysozyme from insect (Manduca sexta) is a cold-adapted enzyme. Protein Pept Lett 14:774–778
Stolarek P, Różalska S, Bernat P (2019) Lipidomic adaptations of the Metarhizium robertsii strain in response to the presence of butyltin compounds. Biochim Biophys Acta—Biomembr 1861:316–326. https://doi.org/10.1016/j.bbamem.2018.06.007
Subbanna ARNS, Stanley J, Venkateswarlu V et al (2019) Toxicological prospects on joint action of microbial insecticides and chemical pesticides. In: Khan MA, Ahmad W (eds) Microbes for sustainable insect pest management. Springer, Cham, pp 317–340. https://doi.org/10.1007/978-3-030-23045-6_12
Supakdamrongkul P, Bhumiratana A, Chanpen W (2010) Characterization of an extracellular lipase from the biocontrol fungus, Nomuraea rileyi MJ, and its toxicity toward Spodoptera litura. J Invertebr Pathol 105:228–235
Szewczyk R, Kuśmierska A, Bernat P (2018) Ametryn removal by Metarhizium brunneum: biodegradation pathway proposal and metabolic background revealed. Chemosphere 190:174–183. https://doi.org/10.1016/j.chemosphere.2017.10.011
Taibon J, Sturm S, Seger C et al (2015) Quantitative assessment of destruxins from strawberry and maize in the lower parts per billion range: combination of a QuEChERS-based extraction protocol with a fast and selective UHPLC-QTOF-MS assay. J Agric Food Chem 63:5707–5713. https://doi.org/10.1021/acs.jafc.5b01562
Tigano-Milani MS, Carneiro RG, De Faria MR et al (1995) Isozyme Characterization and Pathogenicity of Paecilomyces fumosoroseus and P. lilacinus to Diabrotica speciosa (Cleoptera: Chrysomelidae) and Meloidogyne javanica (Nematoda: Tylenchidae). Biol Control 5:378–382
Tkaczuk C, Harasimiuk M, Król A, Beres PK (2015) The effect of selected pesticides on the growth of entomopathogenic fungi Hirsutella nodulosa and Beauveria bassiana. J Ecol Eng 16:177–183. https://doi.org/10.12911/22998993/2952
Tkaczuk C, Majchrowska-Safaryan A, Panasiuk T, Tip** C (2019) Effect of selected heavy metal ions on the growth of entomopathogenic fungi from the Genus Isaria. Appl Ecol Environ Res 17:2571–2582. https://doi.org/10.15666/aeer/1702_25712582
Todorova SI, Coderre D, Duchesne RM, Côté JC (1998) Compatibility of Beauveria bassiana with selected fungicides and herbicides. Environ Entomol 27:427–433. https://doi.org/10.1093/ee/27.2.427
Urbaniak M, Stępień Ł, Uhlig S (2019) Evidence for naturally produced beauvericins containing N-methyl-tyrosine in Hypocreales fungi. Toxins (Basel) 11:182. https://doi.org/10.3390/toxins11030182
Vega FE (2018) The use of fungal entomopathogens as endophytes in biological control: a review. Mycologia 110:4–30. https://doi.org/10.1080/00275514.2017.1418578
Wang C, Leger RJS (2007) The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot Cell 6:808–816. https://doi.org/10.1128/EC.00409-06
Wang Q, Xu L (2012) Beauvericin, a bioactive compound produced by fungi: a short review. Molecules 17:2367–2377. https://doi.org/10.3390/molecules17032367
Wang B, Kang Q, Lu Y et al (2012) Unveiling the biosynthetic puzzle of destruxins in Metarhizium species. Proc Natl Acad Sci 109:1287–1292. https://doi.org/10.1073/pnas.1115983109
Wang H, He Z, Luo L et al (2018a) An aldo-keto reductase, Bbakr1, is involved in stress response and detoxification of heavy metal chromium but not required for virulence in the insect fungal pathogen, Beauveria bassiana. Fungal Genet Biol 111:7–15. https://doi.org/10.1016/j.fgb.2018.01.001
Wang X, Gong X, Li P et al (2018b) Structural diversity and biological activities of cyclic depsipeptides from fungi. Molecules 23:169. https://doi.org/10.3390/molecules23010169
Wang X, Xu J, Wang X et al (2019a) Isaria fumosorosea-based zero-valent iron nanoparticles affect the growth and survival of sweet potato whitefly, Bemisia tabaci (Gennadius). Pest Manag Sci 75:2174–2181
Wang Z, Chen Z, Jiang Z et al (2019b) Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat Commun 10:2538. https://doi.org/10.1038/s41467-019-10386-8
Weng Q, Zhang X, Chen W, Hu Q (2019) Secondary metabolites and the risks of Isaria fumosorosea and Isaria farinosa. Molecules 24(4):pii: E664. https://doi.org/10.3390/molecules24040664
Wu S, Kostromytska OS, Goble T et al (2020) Compatibility of a microsclerotial granular formulation of the entomopathogenic fungus Metarhizium brunneum with fungicides. Biocontrol 9:113–123. https://doi.org/10.1007/s10526-019-09983-9
**a Y, Luo F, Shang Y et al (2017) Fungal cordycepin biosynthesis is coupled with the production of the safeguard molecule pentostatin. Cell Chem Biol 24:1479–1489.e4. https://doi.org/10.1016/j.chembiol.2017.09.001
**ong Z, Wei Q, Chen H et al (2006) Microbial transformation of androst-4-ene-3,17-dione by Beauveria bassiana. Steroids 71:979–983. https://doi.org/10.1016/j.steroids.2006.07.007
Xu Y, Orozco R, Wijeratne EMK et al (2009) Biosynthesis of the cyclooligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveria bassiana. Fungal Genet Biol 46:353–364. https://doi.org/10.1016/j.fgb.2009.03.001
Yang B, Liu H, Yang J et al (2018) New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci Technol 79:116–124. https://doi.org/10.1016/j.tifs.2018.07.006
Yosri M, Abdel-Aziz MM, Sayed RM (2018) Larvicidal potential of irradiated myco-insecticide from Metarhizium anisopliae and larvicidal synergistic effect with its mycosynthesized titanium nanoparticles (TiNPs). J Radiat Res Appl Sci 11:328–334. https://doi.org/10.1016/j.jrras.2018.06.001
Zhang YJ, Liu X, Wang M (2008) Clonig, expression and characterization of two novel cuticle-degrading serine proteases from the entomopathogenic fungus Cordyceps sinensis. Res Microbiol 159:462–469
Zhao H, Lovett B, Fang W (2016) Genetically engineering entomopathogenic fungi. In: Lovett B, Leger RJT (eds) Advances in genetics, vol 94. Elsevier, Amsterdam, pp 137–163
Zimmermann G (2008) The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): biology, ecology and use in biological control. Biocontrol Sci Technol 18:865–901. https://doi.org/10.1080/09583150802471812
Acknowledgements
This research was financed by a grant from the National Science Center in Krakow (Poland), Contract Number UMO-2016/23/B/NZ9/00840.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Litwin, A., Nowak, M. & Różalska, S. Entomopathogenic fungi: unconventional applications. Rev Environ Sci Biotechnol 19, 23–42 (2020). https://doi.org/10.1007/s11157-020-09525-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-020-09525-1