Abstract
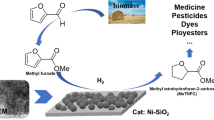
A series of low-cost α-Ni(OH)2/SiO2 catalysts were successfully fabricated by the vapor-induced internal hydrolysis method, then the hydrogenation of 5-hydroxymethylfurfural (HMF) to 2,5-bis(hydroxymethyl)furan (BHMF) was investigated by using the ethanol as the hydrogen donor over these as-prepared α-Ni(OH)2/SiO2 catalysts. Based on the reaction results, these as-prepared catalysts, which could dispense with extra pre-reduction treatment before reactions, exhibited superior catalytic performance for the HMF hydrogenation to BHMF. A remarkable intrinsic selectivity of BHMF selectivity of almost 100% with about 90% HMF conversion could be obtained under 195 °C for 5 h in an N2 atmosphere over the α-Ni(OH)2/SiO2 catalyst of 5-Ni(OH)2/SiO2 with about 5 wt% Ni on SiO2. The almost constant HMF conversion and BHMF intrinsic selectivity found from the recycling tests can indicate that the catalyst was stable without the obvious loss of its catalytic activity after recycling. Moreover, the mechanism of HMF hydrogenation using ethanol over the α-Ni(OH)2/SiO2 catalysts could be referred to as the mechanism of the Meerwein-Ponndorf-Verley reaction. This work will provide a simple synthesis method for future design and development of high-performance, low-cost, and low-energy consumption catalysts for HMF hydrogenation to BHMF.











Similar content being viewed by others
Data Availability
The data has been redescribed.
References
Tang X, Wei J, Ding N, Sun Y, Zeng X, Hu L, Liu S, Lei T, Lin L (2017) Chemoselective hydrogenation of biomass derived 5-hydroxymethylfurfural to diols: key intermediates for sustainable chemicals, materials and fuels. Renew Sust Energ Rev 77:287–296
Hameed S, Lin L, Wang A, Luo W (2020) Recent developments in metal-based catalysts for the catalytic aerobic oxidation of 5-hydroxymethyl-furfural to 2,5-furandicarboxylic acid. Catalysts 10(1):120
Fang W, Riisager A (2021) Recent advances in heterogeneous catalytic transfer hydrogenation/hydrogenolysis for valorization of biomass-derived furanic compounds. Green Chem 23(2):670–688
Wang L, Zuo J, Zhang Q, Peng F, Chen S, Liu Z (2022) Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2,5-dihydroxymethylfuran over Co/UiO-66-NH2. Catal Lett 152(2):361–371
Cao Q, Liang W, Guan J, Wang L, Qu Q, Zhang X, Wang X, Mu X (2014) Catalytic synthesis of 2,5-bis-methoxymethylfuran: a promising cetane number improver for diesel. Appl Catal A: Gen 481:49–53
Alipour S, Omidvarborna H, Kim DS (2017) A review on synthesis of alkoxymethyl furfural, a biofuel candidate. Renew Sust Energ Rev 71:908–926
Zhao W, Wang F, Zhao K, Liu X, Zhu X, Yan L, Yin Y, Xu Q, Yin D (2023) Recent advances in the catalytic production of bio-based diol 2,5-bis(hydroxymethyl)furan. Carbon Resources Convers 6(2):116–131
Alamillo R, Tucker M, Chia M, Pagán-Torres Y, Dumesic J (2012) The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts. Green Chem 14(5):1413–1419
Mishra DK, Lee HJ, Truong CC, Kim J, Suh Y-W, Baek J, Kim YJ (2020) Ru/MnCo2O4 as a catalyst for tunable synthesis of 2,5-bis(hydroxymethyl)furan or 2,5-bis(hydroxymethyl)tetrahydrofuran from hydrogenation of 5-hydroxymethylfurfural. Mol Catal 484:110722
Zhang K, Meng Q, Wu H, He M, Han B (2022) Selective hydrogenolysis of 5-hydroxymethylfurfural into 2,5-dimethylfuran under mild conditions using Pd/MOF-808. ACS Sustain Chem Eng 10(31):10286–10293
Padilla R, Koranchalil S, Nielsen M (2020) Efficient and selective catalytic hydrogenation of furanic aldehydes using well defined Ru and Ir pincer complexes. Green Chem 22(20):6767–6772
Hao W, Li W, Tang X, Zeng X, Sun Y, Liu S, Lin L (2016) Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethyl furfural to the building block 2,5-bishydroxymethyl furan. Green Chem 18(4):1080–1088
Valentini F, Kozell V, Petrucci C, Marrocchi A, Gu Y, Gelman D, Vaccaro L (2019) Formic acid, a biomass-derived source of energy and hydrogen for biomass upgrading. Energ Environ Sci 12(9):2646–2664
Johnson TC, Morris DJ, Wills M (2010) Hydrogen generation from formic acid and alcohols using homogeneous catalysts. Chem Soc Rev 39(1):81–88
Krishnan CK, Hayashi T, Ogura M (2008) A new method for post-synthesis coating of zirconia on the mesopore walls of SBA-15 without pore blocking. Adv Mater 20(11):2131–2136
Wallbridge SP, Lawson K, Catling AE, Kirk CA, Dann SE (2022) Synthesis and spectroscopic identification of nickel and cobalt layered hydroxides and hydroxynitrates. Dalton T 51(47):18010–18023
Xu L, Ding Y-S, Chen C-H, Zhao L, Rimkus C, Joesten R, Suib SL (2008) 3D flowerlike α-nickel hydroxide with enhanced electrochemical activity aynthesized by microwave-assisted hydrothermal method. Chem Mater 20(1):308–316
Rajamathi M, Vishnu Kamath P (1998) On the relationship between α-nickel hydroxide and the basic salts of nickel. J Power Sources 70(1):118–121
Ma Y, Yang M, ** X (2020) Formation mechanisms for hierarchical nickel hydroxide microstructures hydrothermally prepared with different nickel salt precursors. Coll Surface A 588:124374
Park S, Khan Z, Shin TJ, Kim Y, Ko H (2019) Rechargeable Na/Ni batteries based on the Ni(OH)2/NiOOH redox couple with high energy density and good cycling performance. J Mater Chem A 7(4):1564–1573
Hall DS, Lockwood DJ, Poirier S, Bock C, MacDougall BR (2012) Raman and infrared spectroscopy of α and β phases of thin nickel hydroxide films electrochemically formed on nickel. J Phys Chem A 116(25):6771–6784
Xa C, Chen X, Zhang F, Yang Z, Huang S (2013) One-pot hydrothermal synthesis of reduced graphene oxide/carbon nanotube/α-Ni(OH)2 composites for high performance electrochemical supercapacitor. J Power Source 243:555–561
Grosseau-Poussard JL, Dinhut JF, Silvain JF, Sabot R (1999) Role of a chromium ion implantation on the corrosion behaviour of nickel in artificial sea water. Appl Surface Sci 151(1):49–62
Ghesquiere C, Lemaitre J, Herbillon AJ (1982) An investigation of the nature and reducibility of Ni-hydroxy-montmorillonites using various methods including temperature-programmed reduction (TPR). Clay Miner 17(2):217–230
Wang CB, Gau GY, Gau SJ, Tang CW, Bi JL (2005) Preparation and characterization of nanosized nickel oxide. Catal Lett 101(3):241–247
Aghazadeh M, Golikand AN, Ghaemi M (2011) Synthesis, characterization, and electrochemical properties of ultrafine β-Ni(OH)2 nanoparticles. Int J Hydrogen Energ 36(14):8674–8679
Baraldi P, Davolio G, Fabbri G, Manfredini T (1989) A spectral and thermal study on nickel(II) hydroxydes. Mater Chem Phys 21(5):479–493
Clause O, Bonneviot L, Che M (1992) Effect of the preparation method on the thermal stability of silica-supported nickel oxide as studied by EXAFS and TPR techniques. J Catal 138(1):195–205
Zhang C, Zhu W, Li S, Wu G, Ma X, Wang X, Gong J (2013) Sintering-resistant Ni-based reforming catalysts obtained via the nanoconfinement effect. Chem Commun 49(82):9383–9385
Zhang X, Tang W, Zhang Q, Wang T, Ma L (2017) Hydrocarbons production from lignin-derived phenolic compounds over Ni/SiO2 catalyst. Energy Procedia 105:518–523
**a WS, Hou YH, Chang G, Weng WZ, Han GB, Wan HL (2012) Partial oxidation of methane into syngas (H2+CO) over effective high-dispersed Ni/SiO2 catalysts synthesized by a sol-gel method. Int J Hydrogen Energ 37(10):8343–8353
Hu D, Hu H, ** H, Zhang P, Hu Y, Ying S, Li X, Yang Y, Zhang J, Wang L (2020) Building hierarchical zeolite structure by post-synthesis treatment to promote the conversion of furanic molecules into biofuels. Appl Catal A: Gen 590:117338
Acknowledgements
We gratefully acknowledge the Natural Science Foundation of Fujian Province of China (2021J01154), Natural Science Foundation of Academy of Carbon Neutrality of Fujian Normal University (TZH2022-04) for our funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, W., Chen, H. & Chen, A. Selective transformation of biomass-derived 5-hydroxymethylfurfural to the building blocks 2,5-bis(hydroxymethyl)furan on α-Ni(OH)2/SiO2 catalyst without prereduction. Reac Kinet Mech Cat 137, 1635–1649 (2024). https://doi.org/10.1007/s11144-024-02587-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-024-02587-0