Abstract
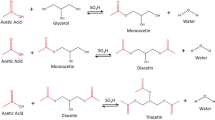
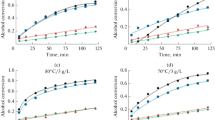
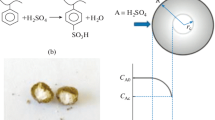
The present work reports a kinetic comparison between two resins made with different cross-linkers, Amberlyst 36 and PS-TMPTA. The latter was synthesized in this study. Both resins were used as catalysts in glycerol acetylation in a batch reactor at 80 and 90 °C, with 5 and 10 g L−1 of catalyst concentration under a molar ratio 4:1 of acetic acid/glycerol. The synthesized resin (PS-TMPTA) presented low ion exchange capacity (1.5 mmol g−1) compared to the commercial resin (Amberlyst 36, 5.45 mmol g−1), but both presented similar efficiency in catalysis, probably due to the difference in cross-linking densities. The experimental results explain the resins’ behavior and properties in detail (ion exchange capacities, swelling index and catalytic efficiency) and the kinetic models were compared utilizing the difference between the corrected Akaike Information Criterion (ΔAICc), Standard Deviation (s) and P-value (student t distribution). According to the results, the irreversible first order model had the best fit of the two models for the experimental conditions studied for this work.




Similar content being viewed by others
References
Galan MI, Bonet J, Sire R et al (2009) (2009) From residual to useful oil: revalorization of glycerine from the biodiesel synthesis. Bioresour Technol 100(15):3775–3778. https://doi.org/10.1016/j.biortech.2009.01.066
Simasatitkula L, Arpornwichanop A (2019) Feasibility study of using waste cooking oil and byproduct from palm oil refinery for green diesel production. Chem Eng Trans. https://doi.org/10.3303/CET1974001
Okoye PU, Abdullah AZ, Hameed BH (2017) Synthesis of oxygenated fuel additives via glycerol esterification with acetic acid over bio-derived carbon catalyst. Fuel 209:538–544. https://doi.org/10.1016/j.fuel.2017.08.024
Tran TTV, Obpirompoo M, Kongparakul S et al (2020) Glycerol valorization through production of di-glyceryl butyl ether with sulfonic acid functionalized KIT-6 catalyst. Carbon Resour Convers 3:182–189. https://doi.org/10.1016/j.crcon.2020.12.003
Liao X, Zhu Y, Wang SG et al (2009) Producing triacetylglycerol with glycerol by two steps: esterification and acetylation. Fuel Process Technol 90(7–8):988–993. https://doi.org/10.1016/j.fuproc.2009.03.015
Setyaningsih L, Siddiq F, Pramezy A (2018) Esterification of glycerol with acetic acid over Lewatit catalyst. MATEC Web Conf 154:01028. https://doi.org/10.1051/matecconf/201815401028
Zhou L, Nguyen TH (2012) Adesina AA (2012) The acetylation of glycerol over amberlyst-15: kinetic and product distribution. Fuel Process Technol 104:310–318. https://doi.org/10.1016/j.fuproc.2012.06.001
Banu I, Bumbac G, Bombos D et al (2020) Glycerol acetylation with acetic acid over Purolite CT-275. Product yields and process kinetics. Renew Energy 148:548–557. https://doi.org/10.1016/j.renene.2019.10.060
Carpegiani JA, Godoy WM, Guimarães DHP, Aguiar LG (2020) Glycerol acetylation catalyzed by an acidic styrene-co-dimethacrylate resin: experiments and kinetic modeling. React Kinet Mech Cat 130:447–461. https://doi.org/10.1007/s11144-020-01788-7
Mekala M, Thamida SK (2013) Goli VL (2013) Pore diffusion model to predict the kinetics of heterogeneous catalytic esterification of acetic acid and methanol. Chem Eng Sci 104:565–573. https://doi.org/10.1016/j.ces.2013.09.039
Godoy WM, Castro G, Nápolis L, Carpegiani JA et al (2020) Synthesis of sulfonated Poly[Styrene-co-(Trimethylolpropane Triacrylate)] and application in the catalysis of glycerol acetylation. Macromol Symp 394(1):1900169. https://doi.org/10.1002/masy.201900169
Penariol JL, Theodoro TR, Dias JR et al (2019) application of a sulfonated styrene–(Ethylene Glycol Dimethacrylate) resin as catalyst. Kinet Catal 60(5):650–653. https://doi.org/10.1134/S0023158419050057
Silva VFL, Penariol JL, Dias JR et al (2019) Sulfonated Styrene-Dimethacrylate resins with improved catalytic activity. Kinet Catal 60(5):654–660. https://doi.org/10.1134/S0023158419050112
Gomes FM, Pereira FM, Silva A (2019) Multiple response optimization: analysis of genetic programming for symbolic regression and assessment of desirability functions. Knowl-Based Syst 179:21–33. https://doi.org/10.1016/j.knosys.2019.05.002
Mufrodi Z, Rochmadi S, Budiman A (2012) Chemical kinetics for synthesis of triacetin from biodiesel byproduct. Int J Chem 4(2):101–107. https://doi.org/10.5539/ijc.v4n2p101
Aguiar L, Moura JOV, Theodoro TR (2017) Prediction of resin textural properties by vinyl/divinyl copolymerization modeling. Polymer 129:21–31. https://doi.org/10.1016/j.polymer.2017.09.042
Coutinho FMB, Rezende SM (2006) Soares BG (2006) Characterization of sulfonated poly (styrene-divinylbenzene) and poly(divinylbenzene) and its application as catalysts in esterification reaction. J Appl Polym Sci 102(4):3616–3627. https://doi.org/10.1002/app.24046
Kunin R (1972) Ion Exchange Resins. Robert E Krieger Publ Co, New York
Theodoro TR, Moura JOV, Dias JR et al (2021) Mathematical modeling of Poly[styrene-co-(ethylene glycol dimethacrylate)] sulfonation. Kinet Catal 62(1):188–195. https://doi.org/10.1134/S0023158421010092
Ferreira P, Fonseca IM, Ramos AM (2009) Esterification of glycerol with acetic acid over dodecamolybdophosphoric acid encaged in USY zeolite. Catal Commun 10(5):481–484. https://doi.org/10.1016/j.catcom.2008.10.015
Fogler HS (2006) Elements of chemical reaction engineering, 4th edn. Prentice Hall PTR, Upper Saddle River
Luenberger DG, Ye Y (2016) Linear and nonlinear programming, 4th edn. Springer International Publishing, New Jersey
Montgomery D, Runger GC (2018) Applied statistics and probability for engineers, 7th edn. Wiley, Hoboken
Aguiar LG, Godoy WM, Nápolis L et al (2021) Modeling the effect of cross-link density on resins catalytic activities. Ind Eng Chem Res 60(17):6101–6110. https://doi.org/10.1021/acs.iecr.1c0095
Moreira MN, Corrêa I, Ribeiro AM et al (2020) Solketal production in a fixed bed adsorptive reactor through the ketalization of glycerol. Ind Eng Chem Res 59(7):2805–2816. https://doi.org/10.1021/acs.iecr.9b06547
Acknowledgements
The authors would like to thank FAPESP (Grant No 2017/26985-4) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Godoy, W.M., Carpegiani, J.A., Pereira, F.M. et al. Kinetic modeling of glycerol acetylation catalyzed by styrene–divinylbenzene and styrene-trimethylolpropane triacrylate sulfonated resins. Reac Kinet Mech Cat 135, 233–245 (2022). https://doi.org/10.1007/s11144-021-02141-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02141-2