Abstract
Aims
To develop a methodology to study uptake and redistribution by plants of NH4+ from deep soil, applying it to investigate deep root N uptake by cultivated grassland species.
Methods
A slow-release 15NH4+ label adsorbed to clinoptilolite was placed into soil (depth 42 cm) well below the densest root zone in well-established monospecific stands of five grass and two clover species. Species showing a variety of deep rooting patterns, N acquisition strategy, forage qualities, and persistence in hemiboreal conditions were chosen. The label was placed in early spring and tracked throughout one or two growing seasons in two repeated experiments.
Results
After two growing seasons ~ 90% of the label was tracked in the soil and harvested herbage of grasses, less in clovers. Deep N uptake was limited in spring, increased during mid-season, and was strongest in autumn in all species, despite lower herbage yield in autumn. Species differed in ability to recover and maintain 15N in the soil–plant system. In one growing season, Lolium perenne L., Phleum pratense L., Schedonorus pratensis (Huds.) P.Beauv. and Schedonorus arundinaceus (Schreb.) Dumort herbage recovered ~ 65% of the label, Poa pratensis L. 54%, and Trifolium pratense L. and Trifolium repens L. 36–48%. Label transport to topsoil was observed, mainly attributable to plant nutrient redistribution rather than physical diffusion.
Conclusions
The innovative slow-release 15N label enabled tracing species differences and seasonal changes in uptake of NH4+ from deep soil. Among the tall-growing grasses, growth vigor appeared as important for deep N uptake as expected root depth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an increasing demand for forage of high quality and protein concentration, produced with high nitrogen (N) use efficiency (NUE) and reduced N losses. Higher yields with higher protein concentration require abundant N fertilization, which tends to reduce NUE and increase N dissipation to the environment. Mineral N fertilizer is often leached below the plough layer, i.e., the densest root zone, which is roughly 0–20 cm in a typical Norwegian forage grassland (Bleken et al. 2022). Perennial grassland species grown for forage differ in rooting depth (Craine et al. 2001) and in their ability to recover N from deeper soil (Hoekstra et al. 2015; Jumpponen et al. 2002; Pirhofer-Walzl et al. 2013).
A root phenotype is the result of genome, environment and management interactions (Großkinsky et al. 2015), with multiple factors influencing root position and activity (Cahill and McNickle 2011; Hodge 2004). Overall N uptake is seasonal and depends on dry matter (DM) production (e.g. Murphy et al. 2013). It is less clear how deep N uptake changes throughout the growing season (Chen et al. 2016). Many studies have shown that purportedly deep-rooting grassland species can access N from deep soil layers (Hoekstra et al. 2015; Kristensen and Thorup-Kristensen 2004). There are few studies on the relative ability of grassland species to take up nutrients from deeper soil, and we are not aware of field studies exploring how acquisition of deep N changes throughout the season.
Many rooting depth studies apply 15N to the soil as 15NO3−, which is rapidly immobilized or lost by leaching (Kristensen and Thorup-Kristensen 2004). Solutions of 15NH4+, which more readily adsorbs to soil particles, though it is prone to microbial immobilization and nitrification, may somewhat prolong label presence in the study system (Davis et al. 2006). In a unique approach, von Felten et al. (2012) used 15N-enriched plant litter as a slow-release 15N source in pots, harvesting plants up to 11 months later. Otherwise, we are not aware of previous experiments tracking the recovery of 15N over several subsequent harvests after a single application.
To impede microbial immobilization, remineralization and leaching of applied 15N we developed a novel label application method using clinoptilolite, a natural zeolite which adsorbs and desorbs NH4+ via ion exchange with pseudo-second-order kinetics (Milovanović et al. 2015). This provided a fixed-point, slow-release label which allowed us to track uptake of NH4+ from a known depth over several successive harvests. We placed 15N-labeled clinoptilolite in early spring in well-established grassland swards at 42 cm depth, in a naturally compact soil layer markedly below the ploughing depth (20 cm) and densest root zone. We then measured 15N recovered in harvested herbage utilizing isotope ratio mass spectrometry.
In this article we present how deep N uptake in monospecific stands related to purported rooting depth, under a variety of conditions throughout two growing seasons. We compare five forage grasses and two N-fixing clovers chosen for showing a variety of deep rooting patterns, N acquisition strategy, forage qualities, and persistence in hemiboreal conditions. Tall fescue (Schedonorus arundinaceus (Schreb.) Dumort) is considered to be deep-rooting (e.g. Hernandez and Picon-Cochard 2016; Malcolm et al. 2015), drought resistant, and well adapted to Nordic conditions. Perennial ryegrass (Lolium perenne L.) is commonly described as fast establishing and shallow rooting (Finn et al. 2013); it is reputed for its high yields and nutritional value but overwinters poorly in Norway (Østrem et al. 2015). Timothy (Phleum pratense L.) and meadow fescue (Schedonorus pratensis (Huds.) P.Beauv.) are well-adapted and commonly-grown forage species in Norway. Bluegrass (Poa pratensis L.) is more common in pastures and is known for lower yields but high N concentration and a shallow rhizomatous root system. Red clover (Trifolium pratense L.) is reputed to be deep-rooting due to its persistent taproot structure (e.g. Ergon et al. 2016), while white clover (Trifolium repens L.) forms shallow stolons.
The following hypotheses were tested: (1) A single application of 15NH4+-enriched clinoptilolite can be used to effectively trace deep root N uptake over several harvests; (2) Generally, the recovery of deep-placed 15N will correlate with growth vigor, and thus be stronger in the spring and summer than in the autumn; (3a) Species reputed as deeper rooting will recover more 15N from 42 cm depth, and N fixers less. Therefore, the annual recovery of deep-placed 15N by monospecific stands from greatest to least will be: tall fescue, meadow fescue and timothy, perennial ryegrass, red clover, bluegrass, and white clover; (3b) In ryegrass the annual recovery of deep-placed 15N will strongly decline from year to year due to poor winter persistence in high latitudes.
Materials and methods
Site
The experiment was established in an Umbric Epistagnic Retisol (IUSS 2015) at the NMBU research farm in Ås, Norway (59°39′49″N, 10°45′38″E, 69 m a.s.l.). The soil is a stone-free silty loam (~ 31% clay, 44% silt and 25% sand), artificially drained at 1 m depth. The bulk density is low in the top layer (1.15 g cm−3), but below the ploughing depth (20 cm) it increases markedly to 1.5 g cm−3 at 40 cm (Suppl. Table S1). During the growing season from May through September, the mean temperature normal (1991–2020) is 13.8 °C. For October through April, the mean temperature normal is 0.9 °C, while it was 1.6 °C and 1.5 °C during the winters leading to spring 2016 and 2017. During the winter, freeze–thaw events and periods with snow cover are common. The annual precipitation normal is 892 mm, roughly evenly distributed through the year, but periods of drought can occur, especially in early summer due to strong insolation and wind. Weather data are from the nearby weather station Ås (No. SN17850) at 59°39′37.8″ N, 10°46′54.5″ E, 94 m a.s.l. (Wolff et al. 2021, 2018, 2017, 2016).
Crop management and experimental treatments
Monospecific stands of perennial ryegrass (Lolium perenne L. cv. Figgjo), tall fescue (Schedonorus arundinaceus (Schreb.) Dumort. cv. Swaj), meadow fescue (Schedonorus pratensis (Huds.) P.Beauv. cv. Fure), timothy (Phleum pratense L. cv. Grindstad), Kentucky bluegrass (Poa pratensis L.), red clover (Trifolium pratense L. cv. Lea) and white clover (Trifolium repens L. cv. Milkanova) were established in June 2014, on 12 sowing rows per plot, spaced 12 cm apart. The best locally-adapted cultivars for agronomic performance and winter survival were chosen. In the third production year, a de-vegetated treatment was added in four subplots by uprooting all plants prior to label application, and kee** them clear of vegetation. Each treatment was replicated in four or more sowing plots (8 m × 1.5 m) which were fully randomized among several treatments not presented here. The de-vegetated subplots were in four plots at the edges of the trial.
In the following years the swards were harvested three times per year (Table 1): in spring (H1) when 10% of timothy inflorescence started to be visible, in summer (H2) after 600–650 growing degree days (basis temperature 0 °C), and in autumn (H3) around mid-September, following local recommendations for high forage digestibility. All treatments received a moderate N fertilizer dose, in total 200 kg N ha−1 yr−1 applied as NPK 22–3-10, distributed 40% in early spring before H1, 40% after H1 and 20% after H2 (Table 1). Red and white clover seed was inoculated using soil from an organically managed crop rotation.
15N Labeling
Two 15N labeling experiments were established before the onset of growth in the early spring of the second (L1: 26–28 April 2016) and third production years (L2: 18–20 April 2017). Each labeling experiment consisted of four new labeled subplots distributed in separate replicates for each monospecific stand. In most cases different sowing plots were used in L1 and L2, except for bluegrass and white clover for which only four plots were available. At least 5 m distance was maintained between subplots labeled in L1 and L2.
The label was prepared by shaking clinoptilolite for 24 h in a 98 atom percent (AT%) 15NH4Cl solution and dried. The amount of NH4-N adsorbed was determined in subsamples (0.5 g clinoptilolite in 25 mL 2 M KCl).
Each subplot received a dose of 36.0 mg 15N, which translates to 68 mg m−2 of plant sampling area (2.4. 15N recovery by plants and redistribution in the soil). To label subplots, we pre-augured a 4 × 4 array of 16 mm diameter, 0.43 m deep holes centered between sowing rows and spaced 12 cm apart (Fig. 1). The 15N-labeled clinoptilolite was introduced through a PVC pipe to fill ~ 41–43 cm depth (1 g hole−1 in L1 and 0,87 g hole−1 in L2). Dry, finely sieved soil from the same field was used to flush the pipe before removing, and after its removal the hole was filled with more dry soil which was carefully compacted. This was done to prevent preferential root growth, as this clayey soil expands on rewetting.
From left, scheme of a subplot with position of 16 holes (dots) for 15N placement between sowing rows, position of 5 soil subsamples (X) for 15N redistribution, plant harvest area (dashed outlines) and margin area used for assessing the sufficiency of the harvest area. Photograph of 15N-labeled clinoptilolite and vial containing one of 16 doses per plot. Depth and method of placement of clinoptilolite
15N recovery by plants and redistribution in the soil
Herbage above ~ 5 cm stubble height was collected at each harvest (2.2. Crop management and experimental treatments) from five sow rows along 70 cm (or an area of 76 by 70 cm in white clover and bluegrass plots where there were no clear sowing rows) (Fig. 1). The plant sampling area, 0.532 m2, was larger than the 48 × 48 cm area immediately surrounding the 15N label placement; this was done to assure a high recovery of the label (Powlson and Barraclough 1993). To check that the harvest area was adequate, in L1 we also sampled herbage from an adjacent 10 cm wide margin, which was assumed to be representative for the whole perimeter (0.3 m2). All herbage was dried at 40–60 °C under ventilation, chopped, and a subsample was ground and ball-milled.
Passive upward 15N redistribution by diffusion and convection in the soil profile was investigated in the de-vegetated subplots in L2. At labeling, the projections of the 16 clinoptilolite positions in the subplot were marked on the soil surface. Soil was sampled one and five months later to 54 and 60 cm, respectively, with 5 soil cores per subplot taken between the projections of the clinoptilolite positions (Fig. 1) and pooled together.
Approximately 17 months after labeling in L1, redistribution of 15N in the soil profile in the presence of plants was studied to 60 cm depth (Suppl. Table S1), in the same manner as shown in Fig. 1. The thickness of the layers was adjusted to center around the clinoptilolite, also taking into consideration the ploughing depth (20 cm), below which soil organic matter (SOM) decreases abruptly, and the soil becomes very compact. All samples were sieved to 2.0 mm and oven-dried at 60 °C under strong ventilation, and a representative subsample was pulverized in an agate mill prior to 15N analysis.
Ancillary observations: soil mineral N and bulk density
Soil mineral N stock (Nmin) was analyzed in the soil removed when preparing holes before label placement (only at 30–43 cm depth in L1, and 0–20, 20–30 and 30–43 cm depths in L2), and again in autumn (26 October 2016 and 30 October 2017). In the autumn, soil samples were taken from non-labeled areas of the treatment plots using a hydraulic press (0–20, 20–40 and 40–60 cm depths, four soil cores per plot). The soil samples were immediately refrigerated to 4 °C and sieved within two days to 2.0 mm. Sieved samples were either immediately extracted for KCl-extractable NH4+, NO2− and NO3− or immediately frozen and extracted later, adding the extractant before thawing (details in Suppl. Table S2). Soil extracts were frozen and analyzed within one month by colorimetric assay (Doane and Horwáth 2003; Krom 1980) on a Tecan Infinite F50 microplate photometer.
The soil bulk density (BD) was measured through the soil profile to 60 cm depth, using 100 cm3 intact cores taken on 1 November 2017 (Suppl. Table S1).
15N analysis and recovery calculations
Nitrogen amount and 15N abundance were analyzed using a Thermo 1112 HT flash combustion element analyzer, coupled through a Finnigan ConFlo III to a Finnigan Delta Plus XP isotope ratio mass spectrometer (IRMS). Atom percent (AT%) of 15N was corrected for scale and drift using house standards which had been calibrated to the international standards IAEA-N1 and N2, and USGS32 and 34. The N amount was quantified by calibrating the peak area of m/z 28 against known amounts of EDTA.
To calculate excess 15N AT% above natural abundance, we subtracted the AT% of unlabeled samples taken from the same field (timothy, 0.3690 AT%, for the grasses; white clover, 0.3686 AT%, for the clovers; natural abundance by depth for soil samples are given in Suppl. Table S1).
Excess AT% was converted to excess 15N fraction by mass F‰ (mg 15N/(g 14N + g 15N)) as:
15N uptake by aboveground herbage (DM) per area was calculated as:
Since the 15N uptake in the herbage at H2 and H3 depended on the label remaining in the soil after previous harvests, 15N recovery strength (Eq. 3) was calculated plotwise at each harvest, assuming plant uptake into harvested herbage to be the only removal of 15N:
Note that cumulative recovery during a growing season was calculated as the total 15N removal in plant herbage relative to the amount applied.
As an indication for the plants’ ability to utilize Nmin from deeper soil to contribute to their total N acquisition, we estimated the Relative Deep Uptake Index (RDUI) as 15N recovery strength per N yield:
We also estimated Recovery per Dry Matter (RDM) as 15N recovery strength per DM yield:
Regarding the soil samples, the amount of 15N label present in each soil layer of thickness (T, cm) was calculated:
The area (A) was assumed to be 48 × 48 cm evenly spaced around the clinoptilolite placement (thus smaller than the harvest area shown in Fig. 1). The measured bulk density (BD, g cm-3) was interpolated to match the exact depths of soil samples taken (2.5. Ancillary observations: soil mineral N and bulk density).
Plant nutritional N status
To evaluate the grasses’ nutritional N status at the time of harvest, the critical N concentration (Ncrit) for potential growth was estimated plotwise and for each harvest as a negative power function of the DM yield, adapted to Norwegian conditions (Baadshaug and Lantinga 2002, Suppl. Text S1). The N status was then calculated as a ratio of the N concentration measured in the herbage (g N g−1 DM) and the Ncrit estimated for each subplot, indicating N deficiency when < 1. This accounts for the dilution of N concentration in herbage with increased plant size, even under non-limiting N supply, due to a greater fiber concentration.
Statistics
The effect of species on each of the response variables considered was tested by one-way analysis of variance (α = 0.05) applied to the cumulative results (sum of several harvests), separately by harvest or soil sampling event, and to the total amount of 15N accounted for in plants and soil 17 months after labeling (L1). Plots of the residual distribution were used to inspect the adequacy of the model, and homoscedasticity was checked and confirmed for all variables using the HOVTEST = BF option in the MEANS statements of the GLM procedure in the statistical package SAS 9.4 (Statistical Analysis System; SAS Institute, NC, USA). In case of significant treatment effect, post hoc Tukey tests were used for multiple comparisons of the species mean values (α = 0.05). To analyze 15N redistribution in the soil, a simple one-sided t-test was used to test for 15N enrichment. For brevity, ‘significant’ is used for ‘statistically significant’.
Two extremely high soil 15N outlier observations were discarded due to suspected contamination with clinoptilolite (38–46 cm layer in ryegrass) or other source in the lab (0–10 cm layer in tall fescue). No other soil or plant 15N observation was removed from the analysis.
Results
Mineral N in the soil profile in the spring and autumn
In both years, the spring and autumn soil Nmin stock in the grass plots was half or less that of the clover plots, and there were no significant differences among the grasses or the clovers (Suppl. Table S3 A). In L1, the initial spring Nmin stock in the soil layer comprising the 15N label and immediately above it (30–43 cm) was ~ 0.9 g m−2 in the grass and 2.5 g m−2 in the clover treatments, and half as much in the following year (L2). Thus, within the plant sampling area, 15N addition corresponded at most to ~ 15% of the Nmin in the 30–43 cm layer, 2–5% of the total soil Nmin (0–43 cm profile, measured only in L2) before fertilizer application and ~ 0.8% of fertilizer N applied in the spring.
Grasses effectively depleted Nmin, leaving ≲1 g Nmin m−2 in the 0–60 cm soil profile by the end of each growing season. Although clovers depleted Nmin less, they left only 2–3 g Nmin m−2 (Suppl. Table S3 B).
Physical diffusion of 15N and redistribution in the soil profile by plant activity
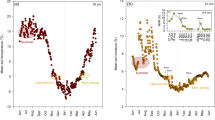
Our approach to follow the herbage uptake of one dose of deep-placed 15N over three harvests assumes minimal upward diffusion of 15N in the absence of plant roots and minimal leaching loss during the growing season. We tested this assumption in the de-vegetated subplots in L2. One month after labeling, at most 3% of the applied 15N was found in the soil layer (38–46 cm depth) surrounding the 15N-loaded clinoptilolite at 42 cm depth, and even less in the adjacent layers below and above the label (Fig. 2A). No significant 15N enrichment was found above 30 cm depth. After 5 months, 7% of the original label was found in the layer immediately below the clinoptilolite position, ~ 4% in the 38–46 cm layer centered around the clinoptilolite, ~ 2% in the layer immediately above it and less than 1% within the ploughing depth (0 to 24 cm, Suppl. Table S4 A). The soil in the vegetated treatments was drier and thus less favorable for Nmin diffusion than in the de-vegetated subplots (data not shown). This indicates that diffusive redistribution was small and that there was little bioturbation upwards was small relative to the 15N uptake by plants.
Recovery of 15N found in each soil layer, avoiding the clinoptilolite placements, relative to the original amount of 15N label placed at ~ 42 cm depth (star). Horizontal lines separate the soil layers sampled, with the 15N recovery shown in the center of the layer. (a) De-vegetated subplots, sampled 1 and 5 months after 15N labeling (L2). A slightly different depth profile was sampled at 1 month because less movement of 15N was expected. 15N enrichment at 0–20 and 20–30 cm depths at 1 month were not significant, and 15N enrichment at combined 0–24 cm depth at 5 months was < 1% of the original label. (b, c) Vegetated subplots, sampled 17 months after 15N labeling (L1). Mean values of 4 replicates; std. errors and p-values from one-sided t-tests are available in Suppl. Table S4
Redistribution of 15N throughout the soil profile due to plant activity was explored in L1 after two growing seasons, i.e. 17 months or six harvests after labeling. On average ~ 20% of the applied 15N was found in the soil profile. Tall fescue subplots retained less 15N in soil than red clover subplots from 0–60 cm depth, and less than meadow fescue subplots at 24–38 cm depth; there were otherwise no significant differences among species (Fig. 2B and C, Suppl. Table S4B). A strong vertical redistribution by plants was evident, as most of the remaining 15N was found in the upper 10 cm of the soil (on average 43%), and nearly 60% in the upper 0–24 cm, while only ~ 12% (1–4% of the 15N applied) was transported below the labeling depth to the 46–60 cm layer. Tall fescue had the least residual soil 15N.
Cumulated over the three successive harvests of L1, only 1.1 to 2% of the original 15N label was recovered in herbage harvested from the 10-cm wide perimeter surrounding the sampling area, the most around tall fescue; no 15N enrichment was detected in the margins of red clover plots (Suppl. Fig. S1). This confirmed that the sampled area was sufficiently large to comprise most of the 15N label recovered in aboveground herbage.
Total 15N accounted for in harvested herbage and soil
After two growing seasons, 17 months after labeling in L1, the sum of 15N in herbage from six consecutive harvests plus that still present in the soil profile accounted for 76–92% of the 15N applied in the subplots of perennial ryegrass, timothy and both fescues (four species hereafter called tall-growing grasses) and red clover (Fig. 3). Less 15N was accounted for in bluegrass (71%) and white clover (57%). Differences were mainly attributed to less 15N in harvested herbage rather than differences in soil 15N (Fig. 3). The 15N in herbage from the fourth through sixth harvests of L1 subplots (the second year after labeling) was small: < 5% of the original 15N label in ryegrass, < 10% in both fescues, up to 10–17% in the remaining species, and was found primarily in the fourth (spring) harvest. These harvests are not included in our evaluation of deep N utilization by different species but are available in Suppl. Table S5.
Percent of 15N label accounted for 17 months after labeling (L1), either in removed herbage of six harvests over two years or found in the soil to a depth of 60 cm. Mean of 4 replicates (± SE). Letters show post hoc Tukey grou**s of the total 15N accounted for. RYE: perennial ryegrass; TIM: timothy; TAL: tall fescue; MEA: meadow fescue; BLU: bluegrass; RC: red clover; WC (not harvested in the first harvest): white clover
Growth conditions and plant performance
The mean temperature during the growing season (May to September) was 14.6 °C and 13.7 °C in 2016 and 2017, respectively. Harsh winters and early spring drought prior to the second (L1) and the third (L2) production years challenged spring (H1) growth of white clover and ryegrass. White clover was not harvested in H1 of L1 due to insufficient growth. Ryegrass had particularly low DM yield in H1 of L2 (Fig. 4A). A summer drought prior to H2 of L2 further reduced yields across species, and annual yields were overall lower in L2 than in L1 (Table 2). A detailed assessment of species response to weather is provided in Suppl. Text S2 and Suppl. Fig. S3.
Per-species and harvest (a) DM yield (g DM m−2), (b) N yield (g N m−2), (c) 15N recovery strength (% recovered of label remaining in soil, Eq. 3), (d) N status of grasses (1 indicates no deficiency, see 2.7. Plant nutritional N status), (e) RDM (15N recovery strength per DM, Eq. 5), (f) RDUI (15N recovery strength per N, Eq. 4). RYE: perennial ryegrass; TIM: timothy; TAL: tall fescue; MEA: meadow fescue; BLU: bluegrass; RC: red clover; WC (not harvested in the first harvest): white clover. Mean of 4 replicates (± SE). See Suppl. Table S6 for post hoc Tukey grou**s and Suppl. Fig. S2 for lines connecting results from each harvest
The growing conditions had a lesser effect on the annual N yield (the amount of total N in the harvested herbage) than on the DM yield, and on average the grasses yielded about 16 and 14.5 g N m−2 which corresponds to 80% and 72% of the fertilizer N applied in L1 and L2 respectively (Table 2). The annual N yield of red clover was about twice as much as that of the grasses (~ 30 g N m−2, 50% higher than the amount of applied fertilizer, indicating a substantial contribution by biological N fixation). White clover yielded less (~ 21 g N m−2) due to a lower DM yield rather than differences in N concentration compared to red clover.
The general decrease in annual DM from L1 to L2 was reflected in all 3 harvests (Fig. 4A). Though there were exceptions pertaining to winter survival and drought response of some species (3.4. Growth conditions and plant performance), the DM yields of the spring and summer harvests were otherwise comparable in size both years. Autumn DM yields were ~ 20–40% lower, and the tall-growing grasses were particularly N-deficient in autumn of both years. The N status (2.7. Plant nutritional N status) of each grass species was similar at each harvest in L2 to the corresponding harvest in L1, with the exception that bluegrass was more N deficient in spring of L2 than in spring of L1 (Fig. 4D).
15N recovery by plant species
The 15N recovery strength, in a contrasting pattern to the general yield levels, was weakest in spring and stronger in the summer and autumn harvests (Fig. 4A, C). In the grasses, 15N recovery strength ranged from 4%-14% in the spring, markedly lower than in the summer (14%-47%) and autumn harvests (14%-35%). In the autumn of L2 all species had much lower 15N recovery than in L1, even though the autumn N yields were similar (Fig. 4B, C).
The strongest 15N-recovering tall-growing grass species shifted from harvest to harvest (Fig. 4C; see Suppl. Table S6 A for post hoc Tukey grou**). In spring of L2 timothy recovered the most 15N together with meadow fescue, while there were no significant species effects in spring of L1. In the summer harvests ryegrass had the highest 15N recovery strength in both years, a position shared with timothy in summer of L2. In the autumn harvests there were no marked differences between the tall-growing grasses, although timothy tended to have the highest 15N recovery strength in L1 and meadow fescue showed a similar tendency in L2. Tall fescue did not have the highest 15N recovery strength in any harvest. The 15N recovery of bluegrass was generally between that of grasses and clovers, though in the drought summer of L2 bluegrass had lower 15N recovery strength than the clovers and did not have better N status than the tall-growing grasses (Fig. 4C, D).
The Recovery per Dry Matter (RDM) weights the 15N recovery strength by DM yield (Eq. 5, Fig. 4E). In both autumns and the drought summer of L2, RDM highlights weak 15N recovery despite strong DM yields by tall fescue and strong 15N recovery by timothy.
The Relative Deep Uptake Index (RDUI), which is 15N recovery strength relative to N yield (Eq. 4, Fig. 4F), followed much the same trends as RDM but better distinguished the deep N uptake behavior of the tall-growing grasses from N-rich bluegrass, and from N-fixing clovers (Table 2). RDUI was the same for a given species in both springs and in both summers, but was higher across all species in autumn of L1 than in autumn of L2, when RDUI was only slightly higher than in the summers. This was despite similar DM yields and N status as in the previous autumn (Fig. 4A, D, F). The clovers reached only 15–20% of the maximum RDUI observed in the tall-growing grasses at each harvest (Suppl. Table S6).
The cumulative herbage uptake of deep-placed 15N over three cuts was smaller in L2 than in L1 (Table 2). In both years, the tall-growing grasses recovered the most 15N (~ 60% of applied in L1 and 50% of applied in L2), whereas the clovers recovered about half as much. Bluegrass recovered similar or slightly higher amounts of 15N than the clovers; it also had markedly higher N concentration but lower 15N N−1 than the other grasses. Despite tall fescue being the highest DM-yielding species both years, and being reputed as deep rooting, compared to the other tall-growing grasses it did not recover more 15N, and it tended to have lower 15N DM−1 and 15N N−1. Ryegrass was among the species recovering the most 15N both years, despite its poor spring regrowth. Timothy increased cumulative 15N recovery relative to the other tall-growing grasses in L2 compared to L1, corresponding with its good winter survival and persistence.
Relationship between 15N recovery strength and other performance variables
The 15N recovery strength was not related with plant vigor as indicated by the DM yield across harvests, as the DM yield of the spring harvest was roughly similar to that in the summer, while the 15N recovery strength was much lower (Fig. 4A, C). This was the case for both the clovers and the grasses.
However, plant vigor could be important within a growing period. We checked this within each seasonal period (spring, summer or autumn, each group including L1 and L2) and separately for grasses (5 species) and clovers (2 species) by comparing the correlation of 15N recovery strength with DM yield and other variables. Overall, DM yield predicted 15N recovery strength significantly and better than did N yield, which in turn was a better predictor of 15N recovery strength than N concentration in the herbage, especially in grasses (Suppl. Table S7). However, an analysis of the scatter plots showed that the relationships were not linear, and the pattern changed between harvests (data not shown).
We checked whether the uptake of deep-placed 15N increased with N deficiency. Across harvests and labeling seasons, the 15N recovery strength and the N status of the grasses changed in reverse order (Fig. 4). However, N status was not a good predictor of 15N recovery strength within seasonal periods for the grasses (Suppl. Table S7), and it did not correlate any better with 15N recovery strength than did herbage N concentration. There is necessarily a dependency between N status and plant vigor, which, as seen, was important for 15N uptake.
Discussion
Clinoptilolite as deep 15NH4 + reservoir over successive harvests
Most 15N studies on root uptake of N in grassland species have either injected or buried quick-dispersing solutions of 15NO3− (Kristensen and Thorup-Kristensen 2004), 15NH4+ (Pirhofer-Walzl et al. 2013), double-labeled NH4NO3 (Hoekstra et al. 2015), or 15N-enriched urea (Malcolm et al. 2015). These studies either harvested the plants once and shortly after injection (6 days—Kristensen and Thorup-Kristensen 2004; 10 days—Pirhofer-Walzl et al. 2013; up to 3–4 weeks – Hoekstra et al. 2015), or used multiple applications over a longer study period with multiple harvests as Malcolm et al. (2015). Von Felten et al. (2012) used 15N-enriched plant litter as slow-release 15N source in pots and harvested plants up to 11 months later. Otherwise, we are not aware of previous experiments following the recovery of 15N over several subsequent harvests after a single application.
Our method of using 15NH4+ adsorbed to clinoptilolite is thus a new and complementary tool for the study of root activity, with several advantages. The long study period allowed us to track active 15N uptake from depth (Fig. 4C, Suppl. Table S5) and its redistribution to the upper soil after two growing seasons, likely through shedding of roots and aboveground litter (Fig. 2B, C). The fact that one and five months after application the passive upward diffusion of 15N in absence of vegetation was small (Fig. 2A) supports that the 15N measured in herbage was a real effect of deep (~ 40 cm) root activity in this naturally compact silty clay soil (bulk density 1.54 g cm−3). Although bioturbation by earthworms could also contribute to vertical redistribution of 15N, few signs of earthworm activity, such as casts, were observed. Active uptake by roots will cause a negative gradient and thus enhance the release of 15NH4+ from the clinoptilolite. Where we obtained evidence of 15NH4+ uptake, we can assume that any NO3− leached to the same depth could be recovered even better, due to its higher diffusion and thus likelihood of being transported to the surface of deep roots.
Only 8–21% of the applied 15N was unaccounted for in the soil and herbage of the tall-growing grass subplots after 17 months (Fig. 3). Our estimation accounts for 15N contained in fine roots or remineralized from plant litter present in the soil analyzed, but excludes 15N stored in larger roots, crowns and stubble left after harvesting (height 5 cm), or still adsorbed to the clinoptilolite, which we did not have the opportunity to analyze. Including them would probably raise the level of accounted-for label even higher (Davis et al. 2006).
The fact that after 17 months we only detected 1–4% of the 15N applied to planted subplots at the 46–60 cm soil depth, below the clinoptilolite, and with no significant differences between species (Fig. 2B, C) suggests little loss by diffusion and leaching of NH4+ in this clayey soil. Since at most 2% of the original 15N label was found in the subplot margins (Suppl. Fig. S1), we conclude that the harvested area was sufficiently large to capture the plants’ ability to recover the original label (“negative discard,” Powlson and Barraclough 1993; Kristensen and Thorup-Kristensen 2004). The fact that margins of grass subplots contained some 15N enrichment, while virtually no 15N was detected in the margin of red clover, can be due to contribution by shedding in the grass subplots. Interestingly, there was no indication of horizontal 15N transport outside the subplot through stolons in white clover or rhizomes in bluegrass.
Increased depth of root activity during the growing season
We found that high herbage production was necessary but not sufficient for efficient utilization of NH4+ from the very compact and SOM-poor deep soil, below a well-structured and SOM-rich ploughed layer and dense root zone (Fig. 4A, C). The slow-release 15N label revealed little deep root activity during spring regrowth compared to summer and autumn, in which 2–3 times more 15N was taken up in plant herbage. In grasses tiller death during the winter entails root death. In early spring, root regrowth is dominated by thick nodal roots, while secondary roots, which are thinner and more effective for nutrient capture, start growing later (Chen et al. 2016). Thus roots from new tillers take time to penetrate deeper soil in spring, especially after poor winter survival. This was best exemplified by ryegrass in L2 (Fig. 4C). That white clover and even red clover recovered very little 15N in the spring compared to the summer and autumn harvests might indicate that shedding of active fine roots during winter and progressive active root depth during the growing season occur both in monocot and dicot species, as also observed by von Felten et al. (2012).
Kristensen and Thorup-Kristensen (2004) found a linear relationship between root density and 15N recovery from different depths. Husse et al. (2017) found that shallow-rooting perennial ryegrass and white clover increased deep N uptake more gradually through the growing season than deep-rooting chicory and red clover, similar to our observation with RDUI. Lower soil temperature in spring compared to summer will also reduce root activity as well as NH4+ diffusion (Macduff et al. 1986). In our study, increasing N deficiency from the first to the last harvest may also have stimulated deep root uptake; however, 15N recovery increased much more than N deficiency, indicating that deep root activity was the most important mechanism for the increased recovery of deep-placed 15N as the growing season proceeded (Fig. 4C, D).
That 15N recovery strength often remained constant or decreased from the second to the third harvest (Fig. 4C) may be partly because we did not account for 15N stored in stubble and large roots. This likely overestimated the amount of 15N remaining in the soil after the summer harvest and thus underestimated the recovery strength of deep-placed 15N in autumn. Conversely, it is possible that through shed roots and leaves some 15N already taken up by plants was translocated to the upper soil during summer. A rapid re-uptake would lead to overestimating deep 15N recovery strength in the autumn harvest. For grasses, the amount of 15N recovered in herbage in the autumn harvest of L1 was 1.5 to 3 times the 15N stock translocated to the upper soil (0–24 cm) one year later, 17 months after labeling. This indicates that deep N uptake was still important in the autumn regrowth period. The heavy rain event during the third regrowth period of L2 (Suppl. Fig. S3) may have caused N leaching and/or plant stress, hel** to explain the systematically lower 15N recovery strength in autumn of L2 compared to autumn of L1 (Fig. 4C).
An important conclusion is that approaches to studying deep nutrient uptake must consider that expression of this functional trait is seasonal (Chen et al. 2016). For example, an experiment on deep-placed 15N recovery performed in the spring cannot be used to deduce the recovery of leached N in the autumn.
Species performance
In general and over the whole growing season, the tall-growing grasses exploited deep N equally well (Fig. 4C). Furthermore, after two growing seasons the tall-growing grasses maintained a larger amount of 15N in the plant-soil system than bluegrass and white clover (Fig. 3), and in both springs the soil profile below the clovers contained twice as much mineral N as the grasses, at least partly attributable to N fixation by the clover species (Suppl. Table S3). These major differences between groups of species were expected because of the thinner root system of the grasses compared with that of clovers, and the shallow rooting depth of bluegrass.
Although tall fescue is known for drought tolerance due to deep rooting, it tended to have lower uptake of 15N relative to DM and N yield than other tall grasses, also under summer drought conditions (Fig. 4E, F). This agrees with the observation by Maire et al. (2009) that tall fescue has thick roots adapted for water transport but with low specific root area and low affinity for NH4+, and by Malcolm et al. (2015) that for tall fescue, growth vigor is more important than presence of deep roots for recovery of N from deeper soil, and dry conditions exacerbate its low affinity for NH4+.
Although ryegrass had poor winter survival, the surviving tillers demonstrated high plasticity and ability for vigorous growth in agreement with observations by Hoekstra et al. (2014), high N uptake and high yields per N uptake (Suppl. Table S6). The thin root system of ryegrass may also be advantageous for NH4+ uptake generally. Ryegrass utilized deep N as well or better than other tall-growing grasses, as exemplified in the summer harvest of L1, when it had the highest 15N recovery strength (47%) observed throughout the experiment (Fig. 4C), in agreement with observations by Pirhofer-Walzl et al. (2013), and with observations that ryegrass has high affinity for NH4+ (Maire et al. 2009). This contrasts with our expectations and with the common perception of ryegrass as shallow-rooting (Husse et al. 2017), particularly when compared to chicory (Pirhofer-Walzl et al. 2013; Hoekstra et al. 2015). However, we did not find studies comparing deep root activity of perennial ryegrass with that of tall fescue or other grasses.
Timothy in particular, and also meadow fescue displayed their well-known good winter hardiness (Østrem et al. 2015; Schjelderup et al. 1994) and performed well, with good utilization of deep N per DM and N yields, also under severe summer drought (Fig. 4E, F). Maire et al. (2009) observed that timothy has a weaker affinity for NH4+ than perennial ryegrass but a stronger affinity for NO3− and NH4+ than tall fescue.
As expected, bluegrass utilized less deep N than the other grasses due to both low DM yields and low 15N DM−1 (Fig. 4A, C, E). Considering that bluegrass had N yields m−2 as high as those of other grasses with higher DM yields, this confirms that bluegrass utilized shallower soil to satisfy its N requirements than the tall-growing grasses tested.
That the clovers took up the least deep N, and there were no significant differences in deep N uptake per N yield between white and red clover (Fig. 4F) may indicate that red clover’s persistent taproot is not advantageous for uptake of NH4+ from depth. In the drought-affected summer harvest of L2 red clover had much weaker 15N recovery than perennial ryegrass, despite similar yields and in contrast with findings by Hoekstra et al. (2015) in a first-year sward. Still, the clovers’ recovery of deep-placed 15N in summer and autumn demonstrates that deep N uptake is a part of their N acquisition strategy.
Weighting 15N uptake in herbage by the amount presumably left in the soil (15N recovery strength), and by DM and N yields (RDM and RDUI), helped to identify differences in species’ inherent ability to utilize NH4+ from deeper soil. RDM highlights the weak 15N recovery by tall fescue relative to timothy, and to a lesser extent meadow fescue, during the drought summer and the autumns (Fig. 4E). Variations in growth vigor between these species appeared to affect neither the inherent ability to utilize deep N nor its utilization in proportion to total N uptake (Fig. 4A, F).
RDM and RDUI were markedly lower in autumn of L2 compared to L1, yet showed a similar species effect (Fig. 4E, F). It is possible that N deficiency caused by the preceding drought, stunting growth equally in the grasses, caused plants to reduce deep root activity (analogous to Hofer et al. 2017), or that less Nmin was transported downward in the soil profile during the drought. If drought in L2 had reduced photosynthesis more than N uptake, we would have expected higher N status in grasses than in the summer harvest of the first year, but this was not the case.
Conclusions
Our method of placing 15NH4+ adsorbed to clinoptilolite at 42 cm depth, as a slow-release label, proved to be a successful tool for comparing species’ ability to take up deep soil N during several regrowth periods (Hypothesis 1). It can also be used to investigate changes in root activity along the growing season or following drought events.
Deep N recovery by grasses and clovers was small in the spring harvest despite high growth vigor, and strongest in the autumn despite lower yields, in contrast to Hypothesis 2. This suggests that deep root activity of both the monocot and dicot species can take time to establish after hemiboreal winters. This phenomenon has implications for the recovery of N transported below ploughing depth and deserves further study.
We expected (Hypothesis 3a) that species in monospecific stands would exploit deep-placed 15NH4+ in the order: tall fescue > meadow fescue and timothy > perennial ryegrass > red clover > bluegrass > white clover. This was only confirmed for the groups (tall-growing grasses > bluegrass > clovers). Tall fescue was somewhat less efficient in taking up deep N than expected from its DM yield, likely due to thick roots with low affinity for NH4+. Despite poor winter survival, and contrary to Hypothesis 3b, ryegrass was among the highest 15N-recovering species, also in the second and third production year and under severe summer drought, owing to vigorous growth and 15N uptake in mid- and late summer. Timothy and meadow fescue are presently the most commonly sown species in Norwegian leys due to their winter hardiness and good forage quality. This study shows that they are also a good strategic choice for N utilization throughout the soil profile.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Baadshaug OH, Lantinga EA (2002) ENGNOR, a Grassland Crop Growth Model for High Latitudes. Technical Documentation. (Reports from AUN. Department of Plant Sciences No. 2/2002). Agricultural University of Norway
Bleken MA, Rittl TF, Nadeem S, Hansen S (2022) Roots and other residues from leys with or without red clover: quality and effects on N2O emission factors in a partly frozen soil following autumn ploughing. Sci Total Environ 831:154582. https://doi.org/10.1016/j.scitotenv.2022.154582
Cahill JF, McNickle GG (2011) The behavioral ecology of nutrient foraging by plants. Annu Rev Ecol Evol Syst 42:289–311. https://doi.org/10.1146/annurev-ecolsys-102710-145006
Chen S-M, Lin S, Loges R, Reinsch T, Hasler M, Taube F (2016) Independence of seasonal patterns of root functional traits and rooting strategy of a grass-clover sward from sward age and slurry application. Grass Forage Sci 71:607–621. https://doi.org/10.1111/gfs.12222
Craine JM, Froehle J, Tilman DG, Wedin DA, Chapin FS III (2001) The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos 93:274–285. https://doi.org/10.1034/j.1600-0706.2001.930210.x
Davis JH, Griffith SM, Horwath WR, Steiner JJ, Myrold DD (2006) Fate of Nitrogen-15 in a perennial ryegrass seed field and herbaceous riparian area. Soil Sci Soc Am J 70:909–919. https://doi.org/10.2136/sssaj2005.0223
Doane TA, Horwáth WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36:2713–2722. https://doi.org/10.1081/AL-120024647
Ergon Å, Kirwan L, Bleken MA, Skjelvåg AO, Collins RP, Rognli OA (2016) Species interactions in a grassland mixture under low nitrogen fertilization and two cutting frequencies: 1. dry-matter yield and dynamics of species composition. Grass Forage Sci 71:667–682. https://doi.org/10.1111/gfs.12250
Finn JA, Kirwan L, Connolly J, Sebastià MT, Helgadottir A, Baadshaug OH, Bélanger G, Black A, Brophy C, Collins RP, Čop J, Dalmannsdóttir S, Delgado I, Elgersma A, Fothergill M, Frankow-Lindberg BE, Ghesquiere A, Golinska B, Golinski P, Grieu P, Gustavsson A-M, Höglind M, Huguenin-Elie O, Jørgensen M, Kadziuliene Z, Kurki P, Llurba R, Lunnan T, Porqueddu C, Suter M, Thumm U, Lüscher A (2013) Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: a 3-year continental-scale field experiment. J Appl Ecol 50:365–375. https://doi.org/10.1111/1365-2664.12041
Großkinsky DK, Svensgaard J, Christensen S, Roitsch T (2015) Plant phenomics and the need for physiological phenoty** across scales to narrow the genotype-to-phenotype knowledge gap. EXBOTJ 66:5429–5440. https://doi.org/10.1093/jxb/erv345
Hernandez P, Picon-Cochard C, (2016) Presence of Trifolium repens promotes complementarity of water use and N facilitation in diverse grass mixtures. Front Plant Sci 7. https://doi.org/10.3389/fpls.2016.00538
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24. https://doi.org/10.1111/j.1469-8137.2004.01015.x
Hoekstra NJ, Finn JA, Hofer D, Lüscher A (2014) The effect of drought and interspecific interactions on depth of water uptake in deep- and shallow-rooting grassland species as determined by δ 18O natural abundance. Biogeosciences 11:4493–4506. https://doi.org/10.5194/bg-11-4493-2014
Hoekstra NJ, Suter M, Finn JA, Husse S, Lüscher A (2015) Do belowground vertical niche differences between deep- and shallow-rooted species enhance resource uptake and drought resistance in grassland mixtures? Plant Soil 394:21–34. https://doi.org/10.1007/s11104-014-2352-x
Hofer D, Suter M, Buchmann N, Lüscher A (2017) Nitrogen status of functionally different forage species explains resistance to severe drought and post-drought overcompensation. Agr Ecosyst Environ 236:312–322. https://doi.org/10.1016/j.agee.2016.11.022
Husse S, Lüscher A, Buchmann N, Hoekstra NJ, Huguenin-Elie O (2017) Effects of mixing forage species contrasting in vertical and temporal nutrient capture on nutrient yields and fertilizer recovery in productive grasslands. Plant Soil 420:505–521. https://doi.org/10.1007/s11104-017-3372-0
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps, World Soil Resources Reports No. 106. FAO, Rome
Jumpponen A, Högberg P, Huss-Danell K, Mulder CPH (2002) Interspecific and spatial differences in nitrogen uptake in monocultures and two-species mixtures in north European grasslands: Nitrogen uptake in north European grasslands. Funct Ecol 16:454–461. https://doi.org/10.1046/j.1365-2435.2002.00642.x
Kristensen HL, Thorup-Kristensen K (2004) Root growth and nitrate uptake of three different catch crops in deep soil layers. Soil Sci Soc Am J 68:529. https://doi.org/10.2136/sssaj2004.0529
Krom MD (1980) Spectrophotometric determination of ammonia: a study of a modified Berthelot reaction using salicylate and dichloroisocyanurate. Analyst 105:305. https://doi.org/10.1039/an9800500305
Macduff JH, Wild A, Hopper MJ, Dhanoa MS (1986) Effects of temperature on parameters of root growth relevant to nutrient uptake: Measurements on oilseed rape and barley grown in flowing nutrient solution. Plant Soil 94:321–332. https://doi.org/10.1007/BF02374326
Maire V, Gross N, da Silveira Pontes L, Picon-Cochard C, Soussana J-F (2009) Trade-off between root nitrogen acquisition and shoot nitrogen utilization across 13 co-occurring pasture grass species. Funct Ecol 23:668–679. https://doi.org/10.1111/j.1365-2435.2009.01557.x
Malcolm BJ, Moir JL, Cameron KC, Di HJ, Edwards GR (2015) Influence of plant growth and root architecture of Italian ryegrass (Lolium multiflorum) and tall fescue (Festuca arundinacea) on N recovery during winter. Grass Forage Sci 70:600–610. https://doi.org/10.1111/gfs.12157
Milovanović J, Eich-Greatorex S, Krogstad T, Rakic V, Rajic N (2015) The use in grass production of clinoptilolite as an ammonia adsorbent and a nitrogen carrier. J Serb Chem Soc 80:1203–1214. https://doi.org/10.2298/JSC150317042M
Murphy PNC, O’Connell K, Watson S, Watson CJ, Humphreys J (2013) Seasonality of nitrogen uptake, apparent recovery of fertilizer nitrogen and background nitrogen supply in two Irish grassland soils. Irish J Agric Food Res 52:17–38
Østrem L, Rapacz M, Larsen A, Dalmannsdottir S, Jørgensen M (2015) Influences of growth cessation and photoacclimation on winter survival of non-native Lolium-Festuca grasses in high-latitude regions. Environ Exp Bot 111:21–31. https://doi.org/10.1016/j.envexpbot.2014.10.008
Pirhofer-Walzl K, Eriksen J, Rasmussen J, Høgh-Jensen H, Søegaard K, Rasmussen J (2013) Effect of four plant species on soil 15N-access and herbage yield in temporary agricultural grasslands. Plant Soil 371:313–325. https://doi.org/10.1007/s11104-013-1694-0
Powlson DS, Barraclough D (1993) Mineralization and assimilation in soil–plant systems. In: Nitrogen isotope techniques. Elsevier, pp 209–242. https://doi.org/10.1016/B978-0-08-092407-6.50013-4
Schjelderup I, Aastveit AH, Aastveit K (1994) Winter hardiness in marginal populations of timothy. In: Rognli OA, Solberg E, Schjelderup I (eds) Breeding Fodder Crops for Marginal Conditions, Developments in Plant Breeding. Springer Netherlands, Dordrecht, pp 61–68. https://doi.org/10.1007/978-94-011-0966-6_6
von Felten S, Niklaus PA, Scherer-Lorenzen M, Hector A, Buchmann N (2012) Do grassland plant communities profit from N partitioning by soil depth? Ecology 93:2386–2396. https://doi.org/10.1890/11-1439.1
Wolff M, Thue-Hansen V, Grimenes AA (2016) Meteorologiske data for Ås 2015. Feltstasjon for bioklimatiske studier, Sørås, Fakultet for realfag og teknologi, Norges Miljø- og Biovitenskapelige Universitet. Available online: https://www.nmbu.no/fakultet/realtek/laboratorier/bioklim/meteorologiske-data
Wolff M, Thue-Hansen V, Grimenes AA (2017) Meteorologiske data for Ås 2016. Feltstasjon for bioklimatiske studier, Sørås, Fakultet for realfag og teknologi, Norges Miljø- og Biovitenskapelige Universitet. Available online: https://www.nmbu.no/fakultet/realtek/laboratorier/bioklim/meteorologiske-data
Wolff M, Thue-Hansen V, Grimenes AA (2018) Meteorologiske data for Ås 2017. Feltstasjon for bioklimatiske studier, Sørås, Fakultet for realfag og teknologi, Norges Miljø- og Biovitenskapelige Universitet. Available online: https://www.nmbu.no/fakultet/realtek/laboratorier/bioklim/meteorologiske-data
Wolff M, Thue-Hansen V, Grimenes AA (2021) Meteorologiske data for Ås 2020. Feltstasjon for bioklimatiske studier, Sørås, Fakultet for realfag og teknologi, Norges Miljø- og Biovitenskapelige Universitet. Available online: https://www.nmbu.no/fakultet/realtek/laboratorier/bioklim/meteorologiske-data
Acknowledgements
This work was funded in part by the Research council of Norway (project AGROPRO – agronomy for increased food production, 225330/E40), and by the Faculty for Environmental Sciences and Natural Resource Management (MINA) at the Norwegian University of Life Sciences. We gratefully acknowledge contributions by Tore Krogstad to the methodology, and assistance from Trygve Fredriksen, Toril Trædal, Øyvind Peder Vartdal, Prashanta Raut, Ragnhild Vold Karlsen, and Sebastian Patzelt.
Funding
Open access funding provided by Norwegian University of Life Sciences This work was funded in part by the Research council of Norway (project AGROPRO – agronomy for increased food production, 225330/E40), received by Marina A. Bleken on behalf of the Norwegian University of Life Sciences (NMBU). A 3-year doctoral employment period for Erin Byers was granted directly by the Faculty for Environmental Sciences and Natural Resource Management (MINA) at the Norwegian University of Life Sciences.
Author information
Authors and Affiliations
Contributions
Erin Byers carried out field and lab work, performed synthesis / results analyses, and was the primary responsible for writing and editing the manuscript. Marina A. Bleken contributed the conception of the experimental methods and experiment design, initiated the field trial, contributed to field and lab work, results analyses, and was secondary responsible for writing and editing the manuscript. Peter Dörsch contributed to the 15N analysis method via IRMS, and to writing and editing the manuscript. Susanne Eich-Greatorex contributed to conceiving the 15N label application method, and contributed to writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Augusto Franco.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Byers, E., Dörsch, P., Eich-Greatorex, S. et al. Deep N acquisition in cultivated grasslands: Uptake of slow-release 15N-labeled ammonium in hemiboreal monospecific leys. Plant Soil 499, 393–408 (2024). https://doi.org/10.1007/s11104-023-06455-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06455-z