Abstract
Aim
Previously, we showed that sowing density influences root length density (RLD), specific root length (SRL) especially in the topsoil, and shallowness of fine roots of field grown spring barley (Hordeum vulagre L.). Here, we ask which trait components may explain these observed changes.
Method
We grew two spring barley cultivars at contrasting sowing densities in both field trials and rhizotrons, and excavated root crowns and imaged root growth.
Results
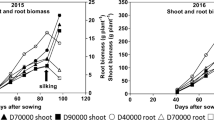
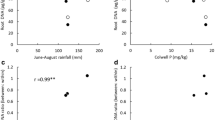
In the field, tiller and nodal root numbers per plant decreased with increasing sowing density, however, nodal roots per tiller, seminal roots per plant, and lateral branching frequencies were not affected. Branching angle did not or only slightly declined with increasing sowing density. In rhizotrons, aboveground only tiller number was affected by sowing density. Root growth rates and counts were not (or only slightly) affected.
Conclusion
Greater RLD at high sowing densities is largely explained by greater main root number per area. The altered seminal to nodal root ratio might explain observed increases in SRL. We conclude that sowing density is a modifier of root system architecture with probable functional consequences, and thereby an important factor to be considered in root studies or the development of root ideotypes for agriculture.







Similar content being viewed by others
Abbreviations
- C_2013:
-
coring in field in 2013 for scanning of roots via NMR
- CKA:
-
Field Lab Campus Klein-Altendorf (field site, research location in Germany)
- D50:
-
depth at which one finds 50% of the total root length [cm]
- DAG:
-
days after germination
- DAS:
-
days after sowing
- Field1_2013:
-
first shovelomics sampling in the field in 2013
- Field2_2013:
-
second shovelomics sampling in the field in 2013
- Field1_2014:
-
first shovelomics sampling in the field in 2014
- Field2_2014:
-
second shovelomics sampling in the field in 2014
- GDD:
-
growing degree days [°C]
- NMR:
-
nuclear magnetic resonance
- MRD:
-
maximum rooting depth (from soil surface to deepest root tip, cm)
- RGR:
-
relative growth rate
- Rhizo1:
-
first experiment in rhizotrons
- Rhizo2:
-
second experiment in rhizotrons
- Rhizo3:
-
third experiment in rhizotrons
- RLD:
-
root length density [root length per unit soil, cm cm−3]
- RSA:
-
root system architecture
- Sowing density:
-
applied treatment, in seeds m−2
- SRL:
-
specific root length [root dry mass per root length, g m−1]
- TRL:
-
total root length (visible at window)
References
Alqudah AM, Schnurbusch T (2015) Barley leaf area and leaf growth rates are maximized during the pre-anthesis phase. Agronomy 5:107–129. https://doi.org/10.3390/agronomy5020107
Amanullah MJH, Nawab K, Ali A (2007) Response of specific leaf area (SLA), leaf area index (LAI) and leaf area ratio (LAR) of maize (Zea mays L.) to plant density, rate and timing of nitrogen application. World Appl Sci J 2:235–243
Anderson-Taylor G, Marshall C (1983) Root-tiller interrelationships in spring barley (Hordeum distichum (L.) Lam.). Ann Bot 51:47–58
Araki H, Iijima M (1998) Rooting nodes of deep roots in rice and maize grown in a long tube. Plant Prod Sci 1:242–247. https://doi.org/10.1626/pps.1.242
Archer E, Strauss HC (1985) Effect of plant density on root distribution of three-year-old grafted 99 Richter grapevines. South Afr Soc Enol Vitic 6:25–30
Archer E, Strauss HC (1989) The effect of plant spacing on the water status of soil and grapevines. South Afr Soc Enol Vitic 10:49–58
Auškalnienė O, Pšibišauskienė G, Auškalnis A, Kadžys A (2010) Cultivar and plant density influence on weediness on spring barley crops. Zemdirbyste-Agriculture 97:53–60
Azam-Ali SN, Gregory PJ, Monteith JL (1984) Effects of planting density on water use and productivity of pearl millet (Pennisetum typhoides) grown on stored water. II. Water use, light interception and dry matter production. Exp Agric 20:215–224
Babe A, Lavigne T, Severin J-P et al (2012) Repression of early lateral root initiation events by transient water deficit in barley and maize. Philos Trans R Soc B Biol Sci 367:1534–1541. https://doi.org/10.1098/rstb.2011.0240
Bade DH, Conrad BE, Holt EC (1985) Temperature and water stress effects on growth of tropical grasses. J Range Manag 38:321–324
Casal JJ, Sanchez RA, Deregibus VA (1986) The effect of plant density on tillering: the involvement of R/FR ratio and the proportion of radiation intercepted per plant. Environ Exp Bot 26:365–371
Castillo CG, Puccio F, Morales D et al (2012) Early arbuscular mycorrhiza colonization of wheat, barley and oats in Andosols of southern Chile. J Soil Sci Plant Nutr 12:511–524
Chen X, Zhang J, Chen Y et al (2013) Changes in root size and distribution in relation to nitrogen accumulation during maize breeding in China. Plant Soil 374:121–130. https://doi.org/10.1007/s11104-013-1872-0
Chochois V, John PV, Greg JR, Watt M (2015) Variation in adult plant phenotypes and partitioning among seed and stem-borne roots across Brachypodium distachyon accessions to exploit in breeding cereals for well-watered and drought environments. Plant Physiol pp 00095.2015. https://doi.org/10.1104/pp.15.00095
Ciampitti IA, Vyn TJ (2011) A comprehensive study of plant density consequences on nitrogen uptake dynamics of maize plants from vegetative to reproductive stages. Field Crop Res 1:2–18
Clark SC (1969) Some effects of temperature and photoperiod on growth and floral development in three winter annuals. New Phytol 68:1137–1144
Darwinkel A (1978) Patterns of tillering and grain production of winter wheat at a wide range of plant densities. Neth J Agric Sci:383–398
Dathe A, Postma JA, Lynch JP, et al (2013) Modeling resource interactions under multiple edaphic stresses. In: Advances in Agricultural Systems Modeling. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America
Davis MH, Simmons SR (1994) Tillering response of barley to shifts in light quality caused by neighboring plants. Crop Sci 34:1604–1610
Demotes-Mainard S, Pellerin S (1992) Effect of mutual shading on the emergence of nodal roots and the root/shoot ratio of maize. Plant Soil 147:87–93. https://doi.org/10.1007/BF00009374
Dornbusch T, Watt J, Baccar R et al (2011) A comparative analysis of leaf shape of wheat, barley and maize using an empirical shape model. Ann Bot 107:865–873. https://doi.org/10.1093/aob/mcq181
Drew MC, Saker LR (1978) Nutrient supply and the growth of the seminal root system in barley III. Compensatory increases in growth of lateral roots, and in rates of phosphate uptake, in response to a localized supply of phosphate. J Exp Bot 29:435–451
Drew MC, Saker LR, Ashley TW (1973) Nutrient supply and the growth of the seminal root system in barley I. The effect of nitrate concentration on the growth of axes and laterals. J Exp Bot 24:1189–1202
Evans JR (1983) Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.). Plant Physiol 72:297–302
Farshbaf-Jafari S, Pirzad A, Tajbakhsh M, Ghassemi-Golezani K (2014) Effects of water supply and plant density on leaf characteristics of Amaranth (Amaranthus caudatus L.). LACSIT Press, Singapore, pp 17–20
Füllner K, Temperton VM, Rascher U et al (2012) Vertical gradient in soil temperature stimulates development and increases biomass accumulation in barley: soil temperature gradient stimulates plant growth. Plant Cell Environ 35:884–892. https://doi.org/10.1111/j.1365-3040.2011.02460.x
Gahoonia TS, Nielsen NE (2004) Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil 262:55–62
Gao Y, Li Y, Zhang J et al (2009) Effects of mulch, N fertilizer, and plant density on wheat yield, wheat nitrogen uptake, and residual soil nitrate in a dryland area of China. Nutr Cycl Agroecosyst 85:109–121. https://doi.org/10.1007/s10705-009-9252-0
Hackett C (1969) A study of the root system of barley II. Relationships between root dimensions and nutrient uptake. New Phytol 1023–1030
Harper JL (1977) Population biology of plants. Academic Press, London
Hecht VL, Temperton VM, Nagel KA et al (2016) Sowing density: a neglected factor fundamentally affecting root distribution and biomass allocation of field grown spring barley (Hordeum vulgare L.). Front Plant Sci 7:1–14. https://doi.org/10.3389/fpls.2016.00944
Holmes MG (1981) Spectral distribution of radiation within plant canopies. In: Smith H (ed) Plants and the daylight spectrum, 1st edn. Academic Press, London, New York, Toronto, Sydney, San Francisco, pp 147–158
Hossain A, Teixeira da Silva JA, Lozovskaya MV, Zvolinsky VP (2012) High temperature combined with drought affect rainfed spring wheat and barley in south-eastern Russia: I. Phenology and growth. Saudi J Biol Sci 19:473–487. https://doi.org/10.1016/j.sjbs.2012.07.005
Hunt WF, Thomas VJ (1985) Growth and developmental responses of perennial ryegrass grown at constant temperature. II. Influence of light and temperature on leaf, tiller and root appearance. Aust J Plant Physiol 12:69–76. https://doi.org/10.1071/PP9850069
Jahnke S, Menzel MI, van Dusschoten D et al (2009) Combined MRI-PET dissects dynamic changes in plant structures and functions. Plant J 59:634–644. https://doi.org/10.1111/j.1365-313X.2009.03888.x
Kamel MS (1959) A physiological study of shading and density effects on the growth and the efficiency of solar energy conversion in some field crops. Wageningen
Kasperbauer MJ (1987) Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiol 85:350–354
Kasperbauer MJ, Karlen DL (1986) Light-mediated bioregulation of tillering and photosynthate partitioning in wheat. Physiol Plant 66:159–163. https://doi.org/10.1111/j.1399-3054.1986.tb01250.x
Kays S, Harper JL (1974) The regulation of plant and tiller density in a grass sward. J Ecol 62:97–105
Khalil SK, Wahab A, Amanullah KAZ (2011) Variation in leaf traits, yield and yield components of faba bean in response to planting dates and densities. Egypt Acad J Biol Sci 2:35–43
Kirby EJM, Appleyard M, Fellowes G (1982) Effect of sowing date on the temperature response of leaf emergence and leaf size in barley. Plant Cell Environ 5:477–484
Knipfer T, Fricke W (2011) Water uptake by seminal and adventitious roots in relation to whole-plant water flow in barley (Hordeum vulgare L.). J Exp Bot 62:717–733. https://doi.org/10.1093/jxb/erq312
Kristensen HL, Thorup-Kristensen K (2009) Roots below one-meter depth are important for uptake of nitrate by annual plants. In: Ma L, Ahuja LR, Bruulsema TW (eds) Quantifying and understanding plant nitrogen uptake for systems modeling, 2? CRC Press, Taylor & Francis Group, Boca Raton, pp 245–258
Kucbel S, Jaloviar P, Špišák J (2011) Quantity, vertical distribution and morphology of fine roots in Norway spruce stands with different stem density. Plant Roots 5:46–55. https://doi.org/10.3117/plantroot.5.46
Kuhlmann H, Barraclough PB (1987) Comparison between the seminal and nodal root systems of winter wheat in their activity for N and K uptake. Z Für Pflanzenernähr Bodenkd 150:24–30
Lafarge TA, Broad IJ, Hammer GL (2002) Tillering in grain sorghum over a wide range of population densities: identification of a common hierarchy for tiller emergence, leaf area development and fertility. Ann Bot 90:87–98
Lancashire PD, Bleiholder H, Boom TVD et al (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol 119:561–601
Landesanstalt für Pflanzenbau und Pflanzenschutz (ed) (2002) Versuchsbericht Sommergerste. Versuchswesen Pflanzenbau Rheinland-Pfalz
Li YS, Yu CB, Zhu S et al (2014) High planting density benefits to mechanized harvest and nitrogen application rates of oilseed rape (Brassica napus L.). Soil Sci Plant Nutr 60:384–392. https://doi.org/10.1080/00380768.2014.895417
Liu S, Song F, Liu F et al (2012) Effect of planting density on root lodging resistance and its relationship to nodal root growth characteristics in maize (Zea mays L.). J Agric Sci 4:182–189. https://doi.org/10.5539/jas.v4n12p182
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112:347–357. https://doi.org/10.1093/aob/mcs293
Manschadi AM, Hammer GL, Christopher JT, deVoil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115–129. https://doi.org/10.1007/s11104-007-9492-1
Marschner H (2012) Marschner’s mineral nutrition of higher plants, 3rd edition. Academic Press, London
McDonald JH (2014) Handbook of biological statistics, 3rd edn. Sparky House Publishing Baltimore, MD, Baltimore, Maryland
McMaster GS, Smika DE (1988) Estimation and evaluation of winter wheat phenology in the central Great Plains. Agric For Meteorol 43:1–18
McMaster GS, Wilhelm WW (1997) Growing degree-days: one equation, two interpretations. Agric For Meteorol 87:291–300
McMaster GS, Wilhelm WW (2003) Phenological responses of wheat and barley to water and temperature: improving simulation models. J Agric Sci 141:129–147. https://doi.org/10.1017/S0021859603003460
Miglietta F (1989) Effect of photoperiod and temperature on leaf initiation rates in wheat (Triticum spp.). Field Crop Res 21:121–130
Miguel MA, Postma JA, Lynch JP (2015) Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiol 167:1430–1439. https://doi.org/10.1104/pp.15.00145
Miller P, Lanier W, Brandt S (2001) Using growing degree days to predict plant stages. Mont State Univ USA Ext Serv
Mommer L, Van Ruijven J, De Caluwe H et al (2010) Unveiling below-ground species abundance in a biodiversity experiment: a test of vertical niche differentiation among grassland species: below-ground species distributions in a biodiversity experiment. J Ecol 98:1117–1127. https://doi.org/10.1111/j.1365-2745.2010.01702.x
Moosavi SG, Seghatoleslami MJ, Moazeni A (2012) Effect of planting date and plant density on morphplogical traits, LAI and forage corn (Sc. 370) yield in second cultivation. Int Res J Appl Basic Sci 3:57–63
Munir AT (2002) Influence of varying seeding rates and nitrogen levels on yield and yield components of barley (Hordeum vulgare L. cv. Rum) in the semi-arid region of Jordan. Bodenkult J Land Manag Food Environ 53:13–18
Nagel KA, Putz A, Gilmer F et al (2012) GROWSCREEN-Rhizo is a novel phenoty** robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Funct Plant Biol 39:891–904
Olsen J, Weiner J (2007) The influence of Triticum aestivum density, sowing pattern and nitrogen fertilization on leaf area index and its spatial variation. Basic Appl Ecol 8:252–257. https://doi.org/10.1016/j.baae.2006.03.013
Oyanagi A, Nakamoto T, Morita S (1993) The gravitropic response of roots and the sha** of the root system in cereal plants. Environ Exp Bot 33:141–158
Paez-Garcia A, Motes C, Scheible W-R et al (2015) Root traits and phenoty** strategies for plant improvement. Plants 4:334–355. https://doi.org/10.3390/plants4020334
Pellerin S (1994) Number of maize nodal roots as affected by plant density and nitrogen fertilization : relationships with shoot growth. Eur J Agron 3:101–110. https://doi.org/10.1016/S1161-0301(14)80115-9
Poorter H, Niklas KJ, Reich PB et al (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50. https://doi.org/10.1111/j.1469-8137.2011.03952.x
Poorter H, Fiorani F, Pieruschka R et al (2016) Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol 212:838–855. https://doi.org/10.1111/nph.14243
Pospišil M, Pospišil A, Rastija M (2000) Effect of plant density and nitrogen rates upon the leaf area of seed sugar beet on seed yield and quality. Eur J Agron 12:69–78
Postma JA, Dathe A, Lynch JP (2014) The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol 166:590–602. https://doi.org/10.1104/pp.113.233916
R Development Core Team (2015) R: a language and environment for statistical computing. R Fouundation for Statistical Computing, Vienna, Austria
Ravenek JM, Bessler H, Engels C et al (2014) Long-term study of root biomass in a biodiversity experiment reveals shifts in diversity effects over time. Oikos 123:1528–1536. https://doi.org/10.1111/oik.01502
Saengwilai P, Tian X, Lynch JP (2014) Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol 166:581–589. https://doi.org/10.1104/pp.113.232603
Schmalenbach I, Pillen K (2009) Detection and verification of malting quality QTLs using wild barley introgression lines. Theor Appl Genet 118:1411–1427. https://doi.org/10.1007/s00122-009-0991-8
Schneider HM, Wojciechowski T, Postma JA et al (2017) Root cortical senescence decreases root respiration, nutrient content and radial water and nutrient transport in barley. Plant Cell Environ 40:1392–1408. https://doi.org/10.1111/pce.12933
Shane MW, Lambers H (2005) Cluster roots: a curiosity in context. Plant Soil 274:101–125
Singh V, van Oosterom EJ, Jordan DR et al (2010a) Morphological and architectural development of root systems in sorghum and maize. Plant Soil 333:287–299. https://doi.org/10.1007/s11104-010-0343-0
Singh V, van Oosterom E, Jordan D, Hammer G (2010b) Genotypic variability for nodal root angle in Sorghum and its implications on potential water extraction. In: Proceedings of the 1st Australian summer grains conference. Gold Coast, Australia, pp 1–10
Soleymani A, Shahrajabian MH, Naranjani L (2011) Determination of the suitable planting date and plant density for different cultivars of barley (Hordeum vulgare L.) in Fars. African J Plant Sci 5:284–286
Su W, Lu J-W, Li X-K, et al (2011) Effect of no-tillage and direct sowing density on growth, nutrient uptake and yield of rapeseed (Brassica napus L.). Sci Agric Sin
Tardieu F (1988) Analysis of the spatial variability of maize root density. Plant Soil 107:259–266
Thorup-Kristensen K (2001) Are differences in root growth of nitrogen catch crops important for their ability to reduce soil nitrate-N content, and how can this be measured? Plant Soil 230:185–195
Thorup-Kristensen K (2006) Effect of deep and shallow root systems on the dynamics of soil inorganic N during 3-year crop rotations. Plant Soil 288:233–248. https://doi.org/10.1007/s11104-006-9110-7
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2010) Shovelomics: high throughput phenoty** of maize (Zea mays L.) root architecture in the field. Plant Soil 341:75–87. https://doi.org/10.1007/s11104-010-0623-8
Turk MA, Tawaha AM, Taifour H et al (2003) Two row barley response to plant density, date of seeding, rate and application of phosphorus in absence of moisture stress. Asian J Plant Sci 2:180–183
Volis S, Shani U (2000) The effect of neighbors on the root system of the desert annual Eremobium aegyptiacum. Folia Geobot 35:161–168. https://doi.org/10.1007/BF02803094
Wahbi A, Gregory PJ (1995) Growth and development of young roots of barley (Hordeum vulgare L.) genotypes. Ann Bot 75:533–539
Wasson AP, Richards RA, Chatrath R et al (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63:3485–3498. https://doi.org/10.1093/jxb/ers111
Weiner J, Thomas SC (1986) Size variability and competition in plant monoculters. Oikos 47:211–222
Weiner J, Stoll P, Muller-Landau H, Jasentuliyana A (2001) The effects of density, spatial pattern, and competitive symmetry on size variation in simulated plant populations. Am Nat 158:438–450
Woolley JT (1971) Reflectance and transmittance of light by leaves. Plant Physiol 47:656–662
Zhan A, Lynch JP (2015) Reduced frequency of lateral root branching improves N capture from low-N soils in maize. J Exp Bot 66:2055–2065. https://doi.org/10.1093/jxb/erv007
Zhan A, Schneider H, Lynch JP (2015) Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol 168:1603–1615. https://doi.org/10.1104/pp.15.00187
Acknowledgements
We acknowledge the diligent farming work at the barley experiment done by the employees of the Campus Klein-Altendorf research station (Germany), especially Winfried Bungert. Further, we thank all the people who helped with sample taking and processing, especially, Jessica Weadow (sample taking and processing), Dagmar van Dusschoten (NMR image acquisition and processing; rhizotron imaging in Rhizo2), Marcel Schneider (sample taking), Christian Kuppe (sample taking), Ann-Kathrin Kleinert (sample taking and processing), Annalena Johnen (sample taking and processing), Anna Galinski (sample taking), Carmen Müller (sample taking), Phil Pstrong (sample taking), Henning Lentz (sample taking), Tanja Goia (sample taking), Jonas Lenz (sample taking), Ines Hecht (sample taking), Igor Lazarevits (sample taking and data acquisition), Simone Schmittgen (sample taking). This research was institutionally funded by the Helmholtz Association (POF III Program—Research Field Key Technologies—Key Technologies for the Bioeconomy).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Kadambot Hamsa Mohamed Siddique
Rights and permissions
About this article
Cite this article
Hecht, V.L., Temperton, V.M., Nagel, K.A. et al. Plant density modifies root system architecture in spring barley (Hordeum vulgare L.) through a change in nodal root number. Plant Soil 439, 179–200 (2019). https://doi.org/10.1007/s11104-018-3764-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3764-9