Abstract
Background and aims
Resistant cultivars are one of the most effective control measures used against bacterial wilt, caused by the soilborne bacterium Ralstonia solanacearum. We aimed to determine the effect of temperature and resistance of tobacco varieties on bacterial wilt occurrence.
Methods
Five tobacco cultivars, with varying resistance levels, were inoculated and transferred to growth chambers at 10, 15, 20, 25, 30, and 35 °C. The growth rate of R. solanacearum was also studied in culture at 10, 20, and 30 °C. The mechanism of resistance was further examined in histological studies conducted at 10, 20, and 30 °C with strain AW1-gfp38 that expresses green fluorescent protein.
Results
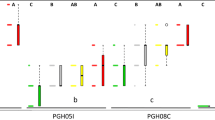
The highest disease incidence was observed at 30 and 35 °C, while no symptoms were observed at 10 and 15 °C. Strains grew in culture at all temperatures. At 30 °C the low resistant cultivars had the most stems colonized by the bacterium, while the highly resistant cultivars mainly had localized infections in the roots.
Conclusions
We suggest that the mechanism of resistance in tobacco is associated with the ability to limit colonization of stem tissues and is temperature dependent. Evaluation of restricted root infections in breeding lines may provide a means for early screening of resistance in breeding programs.






Similar content being viewed by others
References
Aribaud M, Noirot M, Fock-Bastide I, Vanient S, Kodja H (2014) Comparison between Solanum torvum Sw. And S. melongena L. After Ralstonia solanacearum inoculation. Plant Biol 16:1025–1028
Bocsanczy AM, Achenbach UCM, Mangravita-Novo A, Yuen JMF, Norman DJ (2012) Comparative effect of low temperature on virulence and twitching mobility of Ralstonia solanacearum strains present in Florida. Phytopathology 102:185–194
Burk LG, Heggestad HE (1966) The genus Nicotiana: a source of resistance to diseases of cultivated tobacco. Econ Bot 20:76–88
Ciampi L, Sequeira L (1980) Influence of temperature on virulence of race 3 strains of Pseudomonas solanacearum. Am Potato J 57:307–317
Clayton EE, Smith TE (1942) Resistance of tobacco to bacterial wilt (Bacterium solanacearum). J Agric Res 65:547–554
Cruz APZ, Ferreira V, Pianzzola MJ, Siri MI, Coll NS, Valls M (2014) A novel, sensitive method to evaluate potato germplasm for bacterial wilt resistance using a luminescent Ralstonia solanacearum reporter strain. Mol Plant-Micro Int 27:277–285
Dropkin VH (1969) The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne: reversal by temperature. Phytopathology 59:1632–1637
Grimault V, Prior P (1993) Bacterial wilt resistance in tomato associated with tolerance of vascular tissues to Pseudomonas solanacearum. Plant Pathol 42:589–594
Grimault V, Anais G, Prior P (1994) Distribution of Pseudomonas solanacearum in the stem tissues of tomato plants with different levels of resistance to bacterial wilt. Plant Pathol 43:663–668
Hayward AC (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29:65–87
Hendrix JW (1972) Temperature-dependent resistance to tobacco ringspot virus in L8, a necrosis-prone tobacco cultivar. Phytopathology 62:1376–1381
Jablonska B, Ammiraju JS, Bhattarai KK, Mantelin S, Martinez de Iarduya O, Roberts PA, Kaloshian I (2007) The Mi-9 Gene from Solanum arcanum conferring heat-stable resistance to root-knot nematodes is a homolog of Mi-1. Plant Physiol 143:1044–1054
Ji P, Allen C, Sanchez-Perez A, Yao J, Elphinstone JG, Jones JB, Momol MT (2007) New diversity of Ralstonia solanacearum strains associated with vegetable and ornamental crops in Florida. Plant Dis 91:195–203
Katawczik M, Mila AL (2012) Plant age and strain of Ralstonia solanacearum affect the expression of resistance of tobacco cultivars to Granville wilt. Tob Sci 49:8–13
Katawczik M, Tseng HT, Mila AL (2016) Diversity of Ralstonia solanacearum populations affecting tobacco crops in North Carolina. Tob Sci XX (in press)
Kawasaki T, Satsuma H, Fujie M, Usami S, Yamada T (2007) Monitoring of phytopathogenic Ralstonia solanacearum cells using green fluorescent protein-expressing plasmid derived from bacteriophage ΦRSS1. J of Biosci Bioeng 104:451–456
Kelman A (1954) The relationship of pathogenicity in Pseudomonas solanacearum to colony appearance on a tetrazolium medium. Phytopathology 44:693–695
Krausz JP, Thurston HD (1975) Breakdown of resistance to Pseudomonas solanacearum in tomato. Phytopathology 65:1272–1274
Lebeau A, Daunay MC, Frary A, Palloix A, Wang JF, Dintinger J, Chiroleu F, Wicker E, Prior P (2011) Bacterial wilt resistance in tomato, pepper, and eggplant: genetic resources respond to diverse strains in the Ralstonia solanacearum species complex. Phytopathology 101:154–165
Littell R, Milliken GA., Stroup WW, Wolfinger RD, Schabenberber O (2006) SAS for mixed models. Second Edition Cary, NC: SAS Institute Inc
Liu H, Kang Y, Genin S, Schell M, Denny T (2001) Twitching motility of Ralstonia solanacearum requires a type IV pilus system. Microbiology 147:3215–3229
Lucas GB (1975) Diseases of tobacco. 3rd ed. Biological Consulting Associates, Raleigh, NC
Mandal S, Kar I, Mukherjee AK, Acharya P (2013) Elicitor-induced defense responses in Solanum lycopersicum against Ralstonia solanacearum. Sci World J 2013:1–9
McGarvey JA, Denny TP, Schell MA (1999) Spatial-temporal and quantitative analysis of growth and EPS I production by Ralstonia solanacearum in resistant and susceptible tomato cultivars. Phytopathology 89:1233–1239
Mila AL, Radcliff J (2015) Managing diseases. Flue-Cured Tobacco Guide. N. C. Coop. Ext. Serv. Bull., North Carolina State University, Raleigh, In, pp. 124–156
Mori Y, Inoue K, Ikeda K, Nakayashiki H, Higashimoto C, Ohnishi K, Kiba A, and Hikichi Y (2015) The vascular plant-pathogenic bacterium Ralstonia solanacearum produces biofilms required for its virulence on the surfaces of tomato cells adjacent to intercellular spaces. Molecular Plant Path 1–13
Peeters N, Carrère S, Anisimova M, Plener L, Cazalé AC, Genin S (2013) Repertoire, unified nomenclature and evolution of the type III effector gene set in the Ralstonia solanacearum species complex. BMC Genomics 14:859
Powell NT, Melendez PL, Batten CK (1971) Disease complexes in tobacco involving Meloidogyne incognita and certain soil-borne fungi. Phytopathology 61:1332–1337
Prior P, Bart S, Leclercq S, Darrasse A, Anais G (1996) Resistance to bacterial wilt in tomato as discerned by spread of Pseudomonas (Burholderia) solanacearum in the stem tissues. Plant Pathol 45:720–726
Saile E, McGarvey JA, Schell MA, Denny TP (1997) Role of extracellular polysaccharide and endoglucanase in root invasion and colonization of tomato plants by Ralstonia solanacearum. Phytopathology 87:1264–1271
Samuel G (1931) Some experiments on inoculating methods with plant viruses, and on local lesions. Ann Appl Biol 18:494–507
Sierro N, Battey J, Ouadi S, Bakaher N, Bovet L, Willig A, Goepfert S, Peitsch M, Ivanov N (2014) The tobacco genome sequence and its comparison with those of tomato and potato. Nature Communications Article number 3833
Valleau WD (1952) Breeding tobacco for disease resistance. Econ Bot 6:69–102
Vasse J, Frey P, Trigalet A (1995) Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by Pseudomonas solanacearum. Mol Plant Microbe Int 8:241–251
Vasse J, Genin S, Frey P, Boucher C, Brito B (2000) The hrpB and hrpG regulatory genes of Ralstonia solanacearum are required for different stages of the tomato root infection process. Mol Plant Microbe Int 13:259–267
Wang Y, Bao Z, Zhu Y, Hua J (2009) Analysis of temperature modulation of plant defense against biotrophic microbes. Mol Plant Microbe Int 22:498–506
Wei Z, Huang JF, Hu J, Gu YA, Yang CL, Mei XL, Shen QR, Xu YC, Friman VP (2015) Altering transplantation time to avoid periods of high temperature can efficiently reduce bacterial wilt disease incidence with tomato. PLoS One 10:e0139313
Yuliar NYA, Toyota K (2015) Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ 30(1):1–11
Zhang H, Zhang D, Chen J, Yang Y, Huang Z, Huang D, XC W, Huang R (2004) Tomato stress-responsive factor TSRF1 interacts with ethylene responsive element GCC box and regulates pathogen resistance to Ralstonia solanacearum. Plant Mol Biol 55:825–834
Acknowledgments
We would like to thank the North Carolina Tobacco Foundation and the R. J. Reynolds Tobacco Company for their financial support and Dr. Ralph Dean for use of a fluorescent microscope. We would also like to thank Dr. Anjali Iyer-Pascuzzi for review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jesus Mercado-Blanco.
Electronic Supplementary Material
ESM 1
(PDF 154 kb)
Rights and permissions
About this article
Cite this article
Bittner, R.J., Arellano, C. & Mila, A.L. Effect of temperature and resistance of tobacco cultivars to the progression of bacterial wilt, caused by Ralstonia solanacearum . Plant Soil 408, 299–310 (2016). https://doi.org/10.1007/s11104-016-2938-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2938-6