Abstract
Key message
Combining with a CRISPR/Cas9 system, Agrobacterium-mediated transformation can lead to precise targeted T-DNA integration in the rice genome.
Abstract
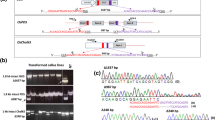
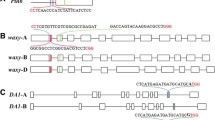
Agrobacterium-mediated T-DNA integration into the plant genomes is random, which often causes variable transgene expression and insertional mutagenesis. Because T-DNA preferentially integrates into double-strand DNA breaks, we adapted a CRISPR/Cas9 system to demonstrate that targeted T-DNA integration can be achieved in the rice genome. Using a standard Agrobacterium binary vector, we constructed a T-DNA that contains a CRISPR/Cas9 system using SpCas9 and a gRNA targeting the exon of the rice AP2 domain-containing protein gene Os01g04020. The T-DNA also carried a red fluorescent protein and a hygromycin resistance (hptII) gene. One version of the vector had hptII expression driven by an OsAct2 promoter. In an effort to detect targeted T-DNA insertion events, we built another T-DNA with a promoterless hptII gene adjacent to the T-DNA right border such that integration of T-DNA into the targeted exon sequence in-frame with the hptII gene would allow hptII expression. Our results showed that these constructs could produce targeted T-DNA insertions with frequencies ranging between 4 and 5.3% of transgenic callus events, in addition to generating a high frequency (50−80%) of targeted indel mutations. Sequencing analyses showed that four out of five sequenced T-DNA/gDNA junctions carry a single copy of full-length T-DNA at the target site. Our results indicate that Agrobacterium-mediated transformation combined with a CRISPR/Cas9 system can efficiently generate targeted T-DNA insertions.


Similar content being viewed by others
References
Ainley WM, Sastry-Dent L, Welter ME, Murray MG, Zeitler B, Amora R et al (2013) Trait stacking via targeted genome editing. Plant Biotechnol J 11:1126–1134
Alonso JM, Stepanova AN, Jeisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Baltes NJ, Voytas DF (2015) Enabling plant synthetic biology through genome engineering. Trends Biotechnol 33:120–131
Bibikova M, Golic M, Golic KG, Carroll D (2002) Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 161:1169–1175
Bochardt A, Hodal L, Palmgren G, Mattsson O, Okkels FT (1992) DNA methylation is involved in maintenance of an unusual expression pattern of an introduced gene. Plant Physiol 99:409–414
Boyle EA, Li YI, Pritchard JK (2017) An expanded view of complex traits: from polygenetic to omnigenic. Cell 15:1177–1186
Breitler JC, Meynard D, Van Boxtel J, Royer M, Bonnot F, Cambillau L, Guiderdoni E (2004) A novel two T-DNA binary vector allows efficient generation of marker-free transgenic plants in three elite cultivars of rice (Oryza sativa L.). Transgenic Res 13:271–287
Brinkman EK, Chen T, Amendola M, van Steensel B (2014) Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res 42:e168
Cai CQ, Doyon Y, Ainley WM, Miller JC, Dekelver RC, Moehle EA et al (2009) Targeted transgene integration in plant cells using designed zinc finger nucleases. Plant Mol Biol 69:699–709
Carroll D (2014) Genome engineering with targetable nucleases. Annu Rev Biochem 83:409–439
Chilton M-D, Que Q (2003) Targeted integration of T-DNA into the tobacco genome at double stranded breaks: New insights on the mechanism of T-DNA integration. Plant Physiol 133:956–965
Clarke R, Heler R, MacDougall MS, Yeo NC, Chavez A, Regan M, Hanakahi L, Church GM, Marraffini LA, Merrill BJ (2018) Enhanced bacterial immunity and mammalian genome editing vir RNA-polymerase-mediated dislodging of Cas9 from double-strand DNA breaks. Mol Cell 71:42–55.e8
Collier R, Thomson JG, Thilmony R (2018) A versatile and robust Agrobacterium-based gene stacking system generates high quality transgenic Arabidopsis plants. Plant J. https://doi.org/10.1111/tpj.13992
Collonnier C, Guyon-Debast A, Maclot F, Mara K, Charlot F, Nogué F (2017) Towards mastering CRISPR/induced gene knock-in in plants: survey of key features and focus on the model Physcomitrella patens. Methods 121–122:103–117
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Dahan-Meir T, Filler-Hayut S, Melamed-Bessudo C, Bocobza S, Czosnek H, Aharoni A, Levy AA (2018) Efficient in planta gene targeting in tomato using gemini viral replicons and the CRISPR/Cas9. Plant J 95:5–16
De Pater S, Pinas JE, Hooykaas PJ, van der Zaal BJ (2013) ZFN-mediated gene targeting of the Arabidopsis protoporphyrinogen oxidase gene through Agrobacterium-mediated floral dip transformation. Plant Biotechnol J 11:510–515
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19:1349
Endo M, Mikami M, Toki S (2015) Multigene knockout utilizing off-target mutations of the CRISPR/Cas9 system in rice. Plant Cell Physiol 56:41–47
Endo M, Mikami M, Toki S (2016) Biallelic gene targeting in rice. Plant Physiol 170:667–677
Field B, Osbourn AE (2008) Metabolic diversification-independent assembly of operon like gene clusters in different plants. Science 320:543–547
Forsyth A, Weeks T, Richael C, Duan H (2016) Transcription activator-like effector nucleases (TALEN)-mediated targeted DNA insertion in potato plants. Front Plant Sci 7:1572. https://doi.org/10.3389/fpls.2016.01572
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67:16–37
Gelvin SB (2017) Integration of Agrobacterium T-DNA into the Plant Genome. Annu Rev Genet 51:195–217
Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345
Hamilton CM, Frary A, Lewis C, Tanksley SD (1996) Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc Natl Acad Sci U S A 93:9975–9979
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hobbs SLA, Kpodar P, DeLong CMO (1990) The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol 15:851–864
Hood EE, Helmer GL, Fraley RT, Chilton MD (1986) The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J Bacteriol 168:1291–1301
Hsiau T, Maures T, Waite K, Yang J, Kelso R, Holden K, Stoner R (2018) Inference of CRISPR edits from Sanger trace data. bioRxiv. https://doi.org/10.1101/251082
Iglesias VA, Moscone EA, Papp I, Neuhuber F, Michalowski S, Phelan T, Spiker S, Matzke M, Matzke AJM (1997) Molecular and cytogenetic analyses of stably and unstably expressed transgene loci in tobacco. Plant Cell 9:1251–1264
Jain M, Nijhawan A, Tyagi AK, Khurana JP (2006) Validation of housekee** genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345:646–651
Jiang W, Zhou H, Bi H, Fromm M, Yang B, Weeks DP (2013) Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res 41:e188
**ek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
**ek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S et al (2014) Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 343:1247997
Kawahara Y, de la Bastide M, Hamilton JP, Kanamori H, McCombie WR, Ouyang S, Schwartz DC, Tanaka T, Wu J, Zhou S, Childs KL, Davidson RM, Lin H, Quesada-Ocampo L, Vaillancourt B, Sakai H, Lee SS, Kim J, Numa H, Itoh T, Buell CR, Matsumoto T (2013) Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice (N Y) 6:4. https://doi.org/10.1186/1939-8433-6-4
Kilby NJ, Leyser HMO, Furner IJ (1992) Promoter methylation and progressive transgene inactivation in Arabidopsis. Plant Mol Biol 20:103–112
Kim H, Kim JS (2014) A guide to genome engineering with programmable nucleases. Nat Rev Genet 15:321–334
Kim SI, Veena, Gelvin SB (2007) Genome-wide analysis of Agrobacterium T-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. Plant J 51:779–791
Kleinboelting N, Huep G, Appelhagen I, Viehoever P, Li Y, Weisshaar B (2015) The structural features of thousands of T-DNA insertion sites are consistent with a double-strand break repair-based insertion mechanism. Mol Plant 8:1651–1654
Köhler F, Cardon G, Pöhlman M, Gill R, Schieder O (1989) Enhancement of transformation rates in higher plants by low-dose irradiation: are DNA repair systems involved in the incorporation of exogenous DNA into the plant genome? Plant Mol Biol 12:189–199
Li JF, Norville JE, Aach J, McCormack M, Zhang D, Bush J, Church GM, Sheen J (2013) Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol 31:688–691
Li J, Meng X, Zong Y, Chen K, Zhang H, Liu J, Li J, Gao C (2016) Gene replacements and insertions in rice by intron targeting using CRISPR-Cas9. Nat Plants 2:16139
Li J, Sun Y, Du J, Zhao Y, **a L (2017) Generation of targeted point mutations in rice by a modified CRISPR/Cas9 system. Mol Plant 6:526–529
Lindsey K, Wei W, Clarke MC, McArdle HF, Rooke LM, Top** JF (1993) Tagging genomic sequences that direct transgene expression by activation of a promoter trap in plants. Transgenic Res 2:33–47
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25:402–408
Main M, Frame B, Wang K (2015) Rice, Japonica (Oryza sativa L.). In: Wang K (ed) Agrobacterium protocols, 3rd edn. Springer, New York, pp 169–180
Matzke AJM, Matzke MA (1998) Position effects and epigenetic silencing of plant transgenes. Curr Opin Plant Biol 1:142–148
McElroy D, Blowers AD, Jenes B, Wu R (1991) Construction of expression vectors based on the rice actin 1 (Act1) 5′ region for use in monocot transformation. Mol Gen Genet 231:150–160. https://doi.org/10.1007/BF00293832
Miranda A, Janssen G, Hodges L, Peralta EG, Reem W (1992) Agrobacterium tumefaciens transfers extremely long T-DNAs by a unidirectional mechanism. J Bacteriol 174:2288–2297
Mlynarova L, Loonen A, Heldens J, Jansen RC, Keizer P, Stiekema WJ, Nap JP (1994) Reduced position effect in mature transgenic plants conferred by the chicken lysozyme matrix-associated region. Plant Cell 6:417–426
Narsai R, Ivanova A, Ng S, Whelan J (2010) Defining reference genes in Oryza sativa using organ, development, biotic and abiotic transcriptome datasets. BMC Plant Biol 10:56. https://doi.org/10.1186/1471-2229-10-56
Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51:1126–1136
Nester EW (2015) Agrobacterium: Nature’s genetic engineer. Front Plant Sci 5:730
Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, Ishitani R, Zhang F, Nureki O (2014) Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156:935–949
Paz MM, Shou H, Guo Z, Zhang Z, Banergee AK, Wang K (2004) Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica 136:67–179
Peach C, Velten J (1991) Transgene expression variability (position effect) of CAT and GUS reporter genes driven by linked divergent T-DNA promoters. Plant Mol Biol 17:49–60
Purnick PE, Weiss R (2009) The second wave of synthetic biology: from modules to systems. Nat Rev Mol Cell Biol 10:410–422
Richardson CD, Ray GJ, DeWitt MA, Curie GL, Corn JE (2016) Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat Biotechnol 34:339–344
Sallaud C, Gay C, Larmande P, Bes M, Piffanelli P, Piegu B, Droc G, Regad F, Bourgeois E, Meynard D et al (2004) High throughput T-DNA insertion mutagenesis in rice: a first step towards in silico reverse genetics. Plant J 39:450–464
Salomon S, Puchta H (1998) Capture of genomic and T-DNA sequences during double-strand break repair in somatic plant cells. EMBO J 17:6086–6095
Sander JD, Joung JK (2014) CRISPR/Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32:347–355
Sasaki T, Matsumoto T, Yamamoto K, Sakata K, Baba T, Katayose Y, Wu J, Niimura Y, Cheng Z, Nagamura Y et al (2002) The genome sequence and structure of rice chromosome 1. Nature 420:312–316
Shan Q, Wang Y, Li J, Zhang Y, Chen K, Liang Z, Zhang K, Liu J, ** JJ, Qiu JL, Gao C (2013) Targeted genome modification of crop plants using a CRISPR/Cas system. Nat Biotechnol 31:686–688
Shukla VK, Doyon Y, Miller JC, DeKelver RC, Moehle EA, Worden SE et al (2009) Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 459:437–441
Svitashev S, Young JK, Schwartz C, Gao H, Falco SC, Cigan AM (2015) Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol 169:931–945
Thole V, Alves SC, Worland B, Bevan MW, Vain P (2009) A protocol for efficiently retrieving and characterizing flanking sequence tags in Brachypodium distachyon T-DNA insertional mutants. Nature Protocol 4:650–661
Townsend JA, Wright DA, Winfrey RJ, Fu F, Maeder ML, Joung JK et al (2009) High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature 459:442–445
Tzfira T, Citovsky V (2006) Agrobacterium-mediated genetic transformation of plants: biology and biotechnology. Curr Opin Biotechnol 17:147–154
Tzfira T, Frankman LR, Vaidya M, Citovsky V (2003) Site-specific integration of Agrobacterium tumefaciens T-DNA via double-stranded intermediates. Plant Physiol 133:1011–1023
Voytas DF, Gao C (2014) Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol 12:e1001877
Wang M, Lu Y, Botella JR, Mao Y, Hua K, Zhu JK (2017) Gene targeting by homology-directed repair in rice using a geminivirus-based CRPSPR/Cas9 system. Mol Plant 10:1007–1010
Wright DA, Townsend JA, Winfrey RJ Jr, Irwin PA, Rajagopal J, Lonosky PM et al (2005) High-frequency homologous recombination in plants mediated by zinc-finger nucleases. Plant J 44:693–705
Yang L, Ding J, Zhang C, Jia J, Weng H, Liu W, Zhang D (2005) Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep 23:759–763
Yin K, Gao C, Qiu JL (2017) Progress and prospects in plant genome editing. Nat Plants 3:17107
Zhang J, Li C, Wu C, **ong L, Chen G, Zhang Q (2006) RMD: a rice mutant database for functional analysis of the rice genome. Nucleic Acids Res 34:D745–D748
Zhang Y, Zhang F, Li X, Baller JA, Qi Y, Starker CG et al (2013) Transcription activator-like effector nucleases enable efficient plant genome engineering. Plant Physiol 161:20–27
Zhang H, Zhang J, Wei P, Zhang B, Gou F, Feng Z, Mao Y, Yang L, Zhang H, Xu N et al (2014) The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol J 12:797–807
Zhang Q, **ng HL, Wang ZP, Zhang HY, Yang F, Wang XC, Chen QJ (2018) Potential high-frequency off-target mutagenesis induced by CRISPR/Cas9 in Arabidopsis and its prevention. Plant Mol Biol 96:445–456
Acknowledgements
We thank David Wright for providing the binary vector pDW3586. This project was partially supported by the Agriculture and Food Research Initiative Competitive Grant no. 2016–06247 from the USDA National Institute of Food and Agriculture (NIFA) to K.W., National Science Foundation Plant Genome Research Program Grant no IOS 1725122 to S.B.G. and K.W., by the USDA NIFA Hatch project # IOW04341, by State of Iowa funds, and by Crop Bioengineering Center of Iowa State University.
Author information
Authors and Affiliations
Contributions
KW, ALE and KL designed the experiments; HZ and MM performed the Agrobacterium-mediated rice transformation; KL, ALE, RB, MEM, and MK analyzed the transgenic plants; KL, ALE, HZ, SBG and KW analyzed the data, and wrote and edited the manuscript. All authors contributed to discussion and revision of the manuscript
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, K., Eggenberger, A.L., Banakar, R. et al. CRISPR/Cas9-mediated targeted T-DNA integration in rice. Plant Mol Biol 99, 317–328 (2019). https://doi.org/10.1007/s11103-018-00819-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-018-00819-1