Abstract
Purpose
Growth hormone (GH)-producing pituitary adenomas (PAs) in childhood or young adulthood are rare, and the details surrounding these tumors remain enigmatic. We present the clinical, pathological and genetic features of this disease.
Methods
We identified 25 patients aged 20 years or younger with GH-producing PAs who underwent surgery between 2003 and 2016 at Toranomon Hospital in Tokyo. We retrospectively reviewed the clinical data, treatment outcomes and pathological features of these patients to shed light on childhood acromegaly.
Results
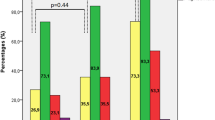
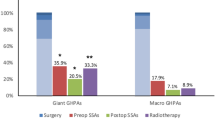
The cohort comprised 14 male and 11 female patients whose average age at the time of surgery was 17.3 years. Germline AIP mutations were present in 5 of 13 patients examined, and Carney complex was identified in 2 of 25 patients. The mean maximum tumor diameter was 26.7 mm, and total resection assessed during surgery was achieved in 17 patients. Based on their respective pathological findings, patients were divided into the following 4 groups: sparsely granulated adenomas (5), densely granulated (DG) adenomas (6), plurihormonal adenomas (9), and silent subtype 3 (SS3) adenomas (5). During the mean follow-up period of 50.3 months, complete endocrinological remission was achieved in 14 of 25 patients (56%) by surgery alone and in 19 patients (76%) after postoperative adjuvant therapy.
Conclusions
GH-producing PAs in young patients are intriguing and difficult to treat due to their distinct tumor characteristics, including a lower incidence of the DG subtype and a higher incidence of SS3 adenomas and genetic abnormalities. Therefore, multi-modal therapies are essential to achieve optimal clinical outcomes.

Similar content being viewed by others
References
Biermasz NR, Smit JWA, Pereira AM, Frölich M, Romijn JA, Roelfsema F (2007) Acromegaly caused by growth hormone-releasing hormone-producing tumors: long-term observational studies in three patients. Pituitary 10:237–249. doi:10.1007/s11102-007-0045-7
Beuschlein F, Strasburger CJ, Siegerstetter V, Moradpour D, Lichter P, Bidlingmaier M, Blum HE, Reincke M (2000) Acromegaly caused by secretion of growth hormone by a non-Hodgkin’s lymphoma. N Engl J Med 342:1871–1876. doi:10.1056/NEJM200006223422504
Kyriakakis N, Trouillas J, Dang MN, Lynch J, Belchetz P, Korbonits M, Murray RD (2017) Diagnostic challenges and management of a patient with acromegaly due to ectopic growth hormone-releasing hormone secretion from a bronchial carcinoid tumour. Endocrinol Diab Metab Case Rep. doi:10.1530/EDM-16-0104
Mehrazin M (2007) Pituitary tumors in children: clinical analysis of 21 cases. Childs Nerv Syst 23:391–398. doi:10.1007/s00381-006-0259-4
Dyer EH, Civit T, Visot A, Delalande O, Derome P (1994) Transsphenoidal surgery for pituitary adenomas in children. Neurosurgery 34:207–212. doi:10.1227/00006123-199402000-00001
Abe T, Tara LA, Lüdecke DK (1999) Growth hormone-secreting pituitary adenomas in childhood and adolescence: features and results of transnasal surgery. Neurosurgery 45:1–10
Nishioka H, Fukuhara N, Horiguchi K, Yamada S (2014) Aggressive transsphenoidal resection of tumors invading the cavernous sinus in patients with acromegaly: predictive factors, strategies, and outcomes. J Neurosurg 121:505–510. doi:10.3171/2014.3.JNS132214
Iwata T, Yamada S, Mizusawa N, Golam HMD, Sano T, Yoshimoto K (2007) The aryl hydrocarbon receptor-interacting protein gene is rarely mutated in sporadic GH-secreting adenomas. Clin Endocrinol 66:499–502. doi:10.1111/j.1365-2265.2007.02758.x
Iwata T, Tamanaha T, Koezuka R, Tochiya M, Makino H, Kishimoto I, Mizusawa N, Ono S, Inoshita N, Yamada S, Shimatsu A, Yoshimoto K (2014) Germline deletion and a somatic mutation of the PRKAR1A gene in a Carney complex-related pituitary adenoma. Eur J Endocrinol 172:K5–K10. doi:10.1530/EJE-14-0685
Albarel F, Castinetti F, Morange I, Conte-Devolx B, Gaudart J, Dufour H, Brue T (2013) Outcome of multimodal therapy in operated acromegalic patients, a study in 115 patients. Clin Endocrinol 78:263–270. doi:10.1111/j.1365-2265.2012.04492.x
Sarkar S, Jacob KS, Pratheesh R, Chacko AG (2014) Transsphenoidal surgery for acromegaly: predicting remission with early postoperative growth hormone assays. Acta Neurochir 156:1379–1387; discussion 1387
Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA (2013) Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab 98:3190–3198. doi:10.1210/jc.2013-1036
Rostomyan L, Daly AF, Petrossians P, Nachev E, Lila AR, Lecoq AL, Lecumberri B, Trivellin G, Salvatori R, Moraitis AG, Holdaway I, Kranenburg-van Klaveren DJ, Chiara Zatelli M, Palacios N, Nozieres C, Zacharin M, Ebeling T, Ojaniemi M, Rozhinskaya L, Verrua E, Jaffrain-Rea ML, Filipponi S, Gusakova D, Pronin V, Bertherat J, Belaya Z, Ilovayskaya I, Sahnoun-Fathallah M, Sievers C, Stalla GK, Castermans E, Caberg JH, Sorkina E, Auriemma RS, Mittal S, Kareva M, Lysy PA, Emy P, De Menis E, Choong CS (2015) Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr Relat Cancer 22:745–757. doi:10.1530/ERC-15-0320
Nomikos P, Buchfelder M, Fahlbusch R (2005) The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure’. Eur J Endocrinol 152:379–387
Kiseljak-Vassiliades K, Carlson NE, Borges MT, Kleinschmidt-DeMasters BK, Lillehei KO, Kerr JM, Wierman ME (2015) Growth hormone tumor histological subtypes predict response to surgical and medical therapy. Endocrine 49:231–241. doi:10.1007/s12020-014-0383-y
Yamada S, Aiba T, Sano T, Kovacs K, Shishiba Y, Sawano S, Takada K (1993) Growth hormone-producing pituitary adenomas: correlations between clinical characteristics and morphology. Neurosurgery 33:20–27
Obari A, Sano T, Ohyama K, Kudo E, Qian ZR, Yoneda A, Rayhan N, Rahman MM, Yamada S (2008) Clinicopathological features of growth hormone-producing pituitary adenomas: difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr Pathol 19:82–91. doi:10.1007/s12022-008-9029-z
Lee CC, Vance ML, Lopes MB, Xu Z, Chen CJ, Sheehan J (2015) Stereotactic radiosurgery for acromegaly: outcomes by adenoma subtype. Pituitary 18:326–334. doi:10.1007/s11102-014-0578-5
Mori R, Inoshita N, Takahashi-Fujigasaki J, Joki T, Nishioka H, Abe T, Fujii T, Yamada S (2013) Clinicopathological features of growth hormone-producing pituitary adenomas in 242 acromegaly patients: classification according to hormone production and cytokeratin distribution. ISRN Endocrinol 2013:723432. doi:10.1155/2013/723432
Bhayana S, Booth GL, Asa SL, Kovacs K, Ezzat S (2005) The implication of somatotroph adenoma phenotype to somatostatin analog responsiveness in acromegaly. J Clin Endocrinol Metab 90:6290–6295
Fougner SL, Casar-Borota O, Heck A, Berg JP, Bollerslev J (2012) Adenoma granulation pattern correlates with clinical variables and effect of somatostatin analogue treatment in a large series of patients with acromegaly. Clin Endocrinol 76:96–102. doi:10.1111/j.1365-2265.2011.04163.x
Lopes MBS (2010) Growth hormone-secreting adenomas: pathology and cell biology. Neurosurg Focus 29:E2. doi:10.3171/2010.7.FOCUS10169
Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M (2013) Growth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: a large single center experience. Pituitary 16:490–498. doi:10.1007/s11102-012-0445-1
Horvath E, Kovacs K, Killinger DW, Smyth HS, Platts ME, Singer W (1980) Silent corticotropic adenomas of the human pituitary gland: a histologic, immunocytologic, and ultrastructural study. Am J Pathol 98:617–638
Yamada S, Kovacs K, Horvath E, Aiba T (1991) Morphological study of clinically nonsecreting pituitary adenomas in patients under 40 years of age. J Neurosurg 75:902–905. doi:10.3171/jns.1991.75.6.0902
Yamada S, Ohyama K, Taguchi M, Takeshita A, Morita K, Takano K, Sano T (2007) A study of the correlation between morphological findings and biological activities in clinically nonfunctioning pituitary adenomas. Neurosurgery 61:580–584; discussion 584. doi:10.1227/01.NEU.0000290906.53685.79
Erickson D, Scheithauer B, Atkinson J, Horvath E, Kovacs K, Lloyd RV, Young WF Jr (2009) Silent subtype 3 pituitary adenoma: a clinicopathologic analysis of the Mayo Clinic experience. Clin Endocrinol 71:92–99. doi:10.1111/j.1365-2265.2008.03514.x
Richardson TE, Mathis DA, Mickey BE, Raisanen JM, Burns DK, White CL III, Hatanpaa KJ (2015) Clinical outcome of silent subtype III pituitary adenomas diagnosed by immunohistochemistry. J Neuropathol Exp Neurol 74:1170–1177. doi:10.1097/NEN.0000000000000265
Mete O, Gomez-Hernandez K, Kucharczyk W, Ridout R, Zadeh G, Gentili F, Ezzat S, Asa SL (2016) Silent subtype 3 pituitary adenomas are not always silent and represent poorly differentiated monomorphous plurihormonal Pit-1 lineage adenomas. Mod Pathol 29:131–142. doi:10.1038/modpathol.2015.151
Yamaguchi-Okada M, Inoshita N, Nishioka H, Fukuhara N, Yamada S (2012) Clinicopathological analysis of nonfunctioning pituitary adenomas in patients younger than 25 years of age. J Neurosurg 9:511–516. doi:10.3171/2012.1.PEDS11330
Horvath E, Kovacs K, Smyth HS, Cusimano M, Singer W (2005) Silent adenoma subtype 3 of the pituitary—immunohistochemical and ultrastructural classification: a review of 29 cases. Ultrastruct Pathol 29:511–524. doi:10.1080/01913120500323514
Syro LV, Rotondo F, Serna CA, Ortiz LD, Kovacs K (2017) Pathology of GH-producing pituitary adenomas and GH cell hyperplasia of the pituitary. Pituitary 20:84–92. doi:10.1007/s11102-016-0748-8
Horvath E, Kovacs K (2006) Pathology of acromegaly. Neuroendocrinology 83:161–165. doi:10.1159/000095524
Daly AF, Tichomirowa MA, Petrossians P, Heliövaara E, Jaffrain-Rea ML, Barlier A (2010) Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab 95:E373-83. doi:10.1210/jc.2009-2556
Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TML, Salmela PI, Paschke R, Gündogdu S, De Menis E, Mäkinen MJ, Launonen V, Karhu A, Aaltonen LA (2006) Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 312:1228–1230
Pack SD, Kirschner LS, Pak E, Zhuang Z, Carney JA, Stratakis CA (2000) Genetic and histologic studies of somatomammotropic pituitary tumors in patients with the “complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas” (Carney complex). J Clin Endocrinol Metab 85:3860–3865. doi:10.1210/jcem.85.10.6875
Schernthaner-Reiter MH, Trivellin G, Stratakis CA (2016) MEN1, MEN4, and Carney Complex: pathology and molecular genetics. Neuroendocrinology 103:18–31. doi:10.1159/000371819
Watson JC, Stratakis CA, Bryant-Greenwood PK, Koch CA, Kirschner LS, Nguyen T, Carney JA, Oldfield EH (2000) Neurosurgical implications of Carney complex. J Neurosurg 92:413–418. doi:10.3171/jns.2000.92.3.0413
Acknowledgements
This work was supported in part by a grant from the Foundation for Growth Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with either the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study, formal consent was not required.
Informed consent
Informed consent was obtained from all individual participants who were included in the study.
Rights and permissions
About this article
Cite this article
Nagata, Y., Inoshita, N., Fukuhara, N. et al. Growth hormone-producing pituitary adenomas in childhood and young adulthood: clinical features and outcomes. Pituitary 21, 1–9 (2018). https://doi.org/10.1007/s11102-017-0836-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-017-0836-4