Abstract
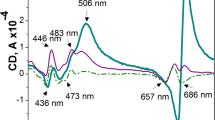
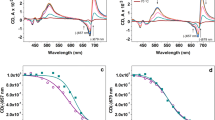
The principal function of the thylakoid membrane depends on the integrity of the lipid bilayer, yet almost half of the thylakoid lipids are of non-bilayer-forming type, whose exact functions are not fully understood. Non-bilayer lipids can be extruded from the membrane in the presence of high concentrations of co-solutes. We applied 2 M sucrose to induce lipid phase separation in isolated thylakoid membranes, following consequent structural and physiological effects. Circular dichroism spectroscopy indicated significant changes in the chiral macro-arrangement of the pigment–protein complexes, which were reversed after washing out the co-solute. Similarly, merocyanine-540 fluorescence suggested reversible changes in the lipid phases. The PSII function, as tested by chlorophyll fluorescence induction transients and time-resolved fluorescence, was almost unaffected. However, the presence of sucrose dramatically increased the PSII thermostability, which can partly be explained by a direct osmolyte effect and partly by the lipid phase separation stabilizing the stacked membrane.
Similar content being viewed by others
Abbreviations
- CD:
-
circular dichroism
- Chl:
-
chlorophyll
- DAES:
-
decay-associated emission spectra
- DGDG:
-
digalactosyldiacylglycerol
- IRF:
-
instrument response function
- MC540:
-
merocyanine-540
- MGDG:
-
monogalactosyl-diacylglycerol
- PG:
-
phosphatidylglycerol
- psi:
-
polymer and salt induced
- SQDG:
-
sulfoquinosyl-diacylglycerol
- TCSPC:
-
time-correlated single-photon counting
References
Akhtar P., Lingvay M., Kiss T. et al.: Excitation energy transfer between light-harvesting complex II and photosystem I in reconstituted membranes.–Biochim. Biophys. Acta 1857: 462–472, 2016.
Barzda V., Istokovics A., Simidjiev I., Garab G.: Structural flexibility of chiral macroaggregates of light-harvesting chlorophyll a/b pigment-protein complexes. Light-induced reversible structural changes associated with energy dissipation.–Biochemistry 35: 8981–8985, 1996.
Bernik D., Tymczyszyn E., Daraio M.E., Negri R.M.: Fluorescent dimers of merocyanine 540 (MC540) in the gel phase of phosphatidylcholine liposomes.–Photochem. Photobiol. 70: 40–48, 1999.
Broess K., Trinkunas G., van Hoek A. et al.: Determination of the excitation migration time in Photosystem II consequences for the membrane organization and charge separation parameters.–Biochim. Biophys. Acta 1777: 404–409, 2008.
Cseh Z., Rajagopal S., Tsonev T. et al.: Thermooptic effect in chloroplast thylakoid membranes. Thermal and light stability of pigment arrays with different levels of structural complexity.–Biochemistry 39: 15250–15257, 2000.
Demé B., Cataye C., Block M.A. et al.: Contribution of galactoglycerolipids to the 3-dimensional architecture of thylakoids.–FASEB J. 28: 3373–3383, 2014.
Douce R., Joyard J.: Biosynthesis of thylakoid membrane lipids.–In: Ort D.R., Yocum C.F. (ed.): Oxygenic Photosynthesis: The Light Reactions. Pp. 69–101. Kluwer Academic Publishers, Dordrecht 1996.
Garab G., Faludi-Daniel A., Sutherland J.C., Hind G.: Macroorganization of chlorophyll a/b light-harvesting complex in thylakoids and aggregates - information from circular differential scattering.–Biochemistry 27: 2425–2430, 1988a.
Garab G., Kieleczawa J., Sutherland J.C. et al: Organization of pigment protein complexes into macrodomains in the thylakoid membranes of wild-type and chlorophyll-b-less mutant of barley as revealed by circular-dichroism.–Photochem. Photobiol. 54: 273–281, 1991.
Garab G., Lohner K., Laggner P., Farkas T.: Self-regulation of the lipid content of membranes by non-bilayer lipids: a hypothesis.–Trends Plant Sci. 5: 489–494, 2000.
Garab G., Ughy B., Goss R.: Role of MGDG and non-bilayer lipid phases in the structure and dynamics of chloroplast thylakoid membranes.–In: Nakamura Y., Li-Beisson Y. (ed.): Lipids in Plant and Algae Development. Pp. 127–157. Springer, Dordrecht 2016.
Garab G., Ughy B., Waard P. et al.: Lipid polymorphism in chloroplast thylakoid membranes-as revealed by (31) P-NMR and time-resolved merocyanine fluorescence spectroscopy.–Sci. Rep. 7: 13343, 2017.
Garab G., van Amerongen H.: Linear dichroism and circular dichroism in photosynthesis research.–Photosynth. Res. 101: 135–146, 2009.
Garab G., Wells S., Finzi L., Bustamante C.: Helically organized macroaggregates of pigment protein complexes in chloroplasts - evidence from circular intensity differential scattering.–Biochemistry 27: 5839–5843, 1988b.
Goltsev V., Yordanov I., Tsonev T.: Evaluation of relative contribution of initial and variable chlorophyll fluorescence measured at different temperatures.–Photosynthetica 30: 629–643, 1994.
Goss R., Lohr M., Latowski D. et al.: Role of hexagonal structure-forming lipids in diadinoxanthin and violaxanthin solubilization and de-epoxidation.–Biochemistry 44: 4028–4036, 2005.
Goss R., Oroszi S., Wilhelm C.: The importance of grana stacking for xanthophyll cycle-dependent NPQ in the thylakoid membranes of higher plants.–Physiol. Plantarum 131: 496–507, 2007.
Holzwarth A.R., Miloslavina Y., Nilkens M., Jahns P.: Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence.–Chem. Phys. Lett. 483: 262–267, 2009.
Jahns P., Latowski D., Strzalka K.: Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids.–BBA-Bioenergetics 1787: 3–14, 2009.
Janik E., Bednarska J., Zubik M. et al.: Molecular architecture of plant thylakoids under physiological and light stress conditions: A study of lipid-light-harvesting complex II model membranes.–Plant Cell 25: 2155–2170, 2013.
Kirchhoff H., Haase W., Wegner S. et al.: Low-light-induced formation of semicrystalline photosystem II arrays in higher plant chloroplast.–Biochemistry 46: 11169–11176, 2007.
Kouřil R., Lazár D., Ilík P. et al.: High-temperature induced chlorophyll fluorescence rise in plants at 40–50 °C: Experimental and theoretical approach.–Photosynth. Res. 81: 49–66, 2004.
Kovács L., Damkjaer J., Kereïche S. et al.: Lack of the lightharvesting complex CP24 affects the structure and function of the grana membranes of higher plant chloroplasts.–Plant Cell 18: 3106–3120, 2006.
Kowalewska L., Mazur R., Suski S. et al.: Three-dimensional visualization of the internal plastid membrane network during runner bean chloroplast biogenesis. Dynamic model of the tubular-lamellar transformation.–Plant Cell 28: 875–891, 2016.
Krishna M., Periasamy N.: Fluorescence of organic dyes in lipid membranes: site of solubilization and effects of viscosity and refractive index on lifetimes.–J. Fluoresc. 8: 81–91, 1998.
Krumova S.B., Dijkema C., de Waard P. et al.: Phase behavior of phosphatidylglycerol in spinach thylakoid membranes as revealed by 31P-NMR.–BBA-Biomembranes 1778: 997–1003, 2008a.
Krumova S.B., Koehorst R.B.M., Bóta A. et al.: Temperature dependence of the lipid packing in thylakoid membranes studied by time- and spectrally resolved fluorescence of Merocyanine.–Biochim. Biophys. Acta 1778: 2823–2833, 2008b.
Krumova S.B., Laptenok S.P., Kovács L. et al.: Digalactosyldiacylglycerol- deficiency lowers the thermal stability of thylakoid membranes.–Photosynth. Res. 105: 229–242, 2010.
Langner M., Hui S.W.: Merocyanine 540 as a fluorescence indicator for molecular packing stress at the onset of lamellarhexagonal transition of phosphatidylethanolamine bilayers.–Biochim. Biophys. Acta 1415: 323–330, 1999.
Latowski D., Akerlund H.E., Strzałka K.: Violaxanthin deepoxidase, the xanthophyll cycle enzyme, requires lipid inverted hexagonal structures for its activity.–Biochemistry 43: 4417–4420, 2004.
Miloslavina Y., Wehner A., Lambrev P.H. et al.: Far-red fluorescence: A direct spectroscopic marker for LHCII oligomers forming in non-photochemical quenching.–FEBS Lett. 582: 3625–3631, 2008.
Mitchell P.: Chemiosmotic coupling in oxidative and photosynthetic phosphorylation.–Biol. Rev. Camb. Philos. 41: 445–501, 1966.
Nagy G., Szabó M., Ünnep R. et al.: Modulation of the multilamellar membrane organization and of the chiral macrodomains in the diatom Phaeodactylum tricornutum revealed by small-angle neutron scattering and circular dichroism spectroscopy.–Photosynth. Res. 111: 71–79, 2012.
Nagy G., Ünnep R., Zsiros O. et al.: Chloroplast remodeling during state transitions in Chlamydomonas reinhardtii as revealed by noninvasive techniques in vivo.–P. Natl. Acad. Sci. USA 111: 5042–5047, 2014.
Pal S.K., Sukul D., Mandal D., Bhattacharyya K.: Solvation dynamics of DCM in lipid.–J. Phys. Chem. B 104: 4529–4531, 2000.
Páli T., Garab G., Horváth L.I., Kóta Z.: Functional significance of the lipid-protein interface in photosynthetic membranes.–Cell Mol. Life Sci. 60: 1591–1606, 2003.
Posselt D., Nagy G., Kirkensgaard J.J. et al.: Small-angle neutron scattering study of the ultrastructure of chloroplast thylakoid membranes–periodicity and structural flexibility of the stroma lamellae.–Biochim. Biophys. Acta 1817: 1220–1228, 2012.
Pueyo J.J., Alfonso M., Andrés C., Picorel R.: Increased tolerance to thermal inactivation of oxygen evolution in spinach Photosystem II membranes by substitution of the extrinsic 33-kDa protein by its homologue from a thermophilic cyanobacterium.–Biochim. Biophys. Acta 1554: 29–35, 2002.
Roelofs T.A., Lee C.-H., Holzwarth A.R.: Global target analysis of picosecond chlorophyll fluorescence kinetics from pea chloroplasts. A new approach to the characterization of the primary processes in photosystem II α- and β-units.–Biophys. J. 61: 1147–1163, 1992.
Simidjiev I., Barzda V., Mustárdy L., Garab G.: Role of thylakoid lipids in the structural flexibility of lamellar aggregates of the isolated light-harvesting chlorophyll a/b complex of photosystem II.–Biochemistry 37: 4169–4173, 1998.
Simidjiev I., Stoylova S., Amenitsch H. et al.: Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro.–P. Natl. Acad. Sci. USA 97: 1473–1476, 2000.
Stirbet A., Govindjee: On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient.–J. Photoch. Photobio. B 104: 236–257, 2011.
Strasser R.J., Tsimilli-Michael M., Srivastava A.: Analysis of the chlorophyll a fluorescence transient.–In: Papageorgiou G.C., Govindjee (ed.): Chlorophyll a Fluorescence: A Signature of Photosynthesis. Pp. 463–495. Springer, Dordrecht 2004.
Stratt R.M., Maroncelli M.: Nonreactive dynamics in solution: The emerging molecular view of solvation dynamics and vibrational relaxation.–J. Phys. Chem. 100: 12981–12996, 1996.
Tóth T.N., Rai N., Solymosi K. et al.: Fingerprinting the macroorganisation of pigment-protein complexes in plant thylakoid membranes in vivo by circular-dichroism spectroscopy.–BBA-Bioenergetics 1857: 1479–1489, 2016.
Tsvetkova N.M., Apostolova E.L., Brain A.P. et al.: Factors influencing PSII particle array formation in Arabidopsis thaliana chloroplasts and the relationship of such arrays to the thermostability of PS II.–BBA-Bioenergetics 1228: 201–210, 1995.
Ünnep R., Zsiros O., Solymosi K. et al.: The ultrastructure and flexibility of thylakoid membranes in leaves and isolated chloroplasts as revealed by small-angle neutron scattering.–BBA-Bioenergetics 1837: 1572–1580, 2014.
van der Weij-de Wit C.D., Ihalainen J.A., van Grondelle R., Dekker J.P.: Excitation energy transfer in native and unstacked thylakoid membranes studied by low temperature and ultrafast fluorescence spectroscopy.–Photosynth. Res. 93: 173–182, 2007.
van Eerden F.J., de Jong D.H., de Vries A.H. et al.: Characterization of thylakoid lipid membranes from cyanobacteria and higher plants by molecular dynamics simulations.–Biochim. Biophys. Acta 1848: 1319–1330, 2015.
Williams W., Brain A., Dominy P.: Induction of non-bilayer lipid phase separations in chloroplast thylakoid membranes by compatible co-solutes and its relation to therthermal stability of Photosystem II.–BBA-Bioenergetics 1099: 137–144, 1992.
Williams W., Gounaris K.: Stabilisation of PS-II-mediated electron transport in oxygen-evolving PS II core preparations by the addition of compatible co-solutes.–Biochim. Biophys. Acta 1100: 92–97, 1992.
Williams W.P.: The physical properties of thylakoid membrane lipids and their relation to photosynthesis.–In: Siegenthaler P.-A., Murata N. (ed.): Lipids in Photosynthesis: Structure, Function and Genetics. Pp. 103–118. Springer, Dordrecht 1998.
Yamamoto H.Y., Higashi R.: Violaxanthin de-epoxidase: lipid composition and substrate specificity.–Arch. Biochem. Biophys. 190: 514–522, 1978.
Yancey P.H.: Compatible and counteracting solutes.–In: Strange K. (ed.): Cellular and Molecular Physiology of Cell Volume Regulation. Pp. 81–109. CRC Press, Boca Raton 1994.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: This work benefitted of grants from the Hungarian Ministry for National Economy (GINOP-2.3.2-15-2016-00001 and GINOP-2.3.2-15-2016-00058) and the National Research Development and Innovation Office of Hungary (OTKA K 112688 to G.G.). C.K. was supported by a postdoctoral fellowship from the Hungarian Academy of Sciences. G.G. acknowledges partial support from Moravian-Silesian Region (Project 01211/2016/RRC).
Rights and permissions
About this article
Cite this article
Kotakis, C., Akhtar, P., Zsiros, O. et al. Increased thermal stability of photosystem II and the macro-organization of thylakoid membranes, induced by co-solutes, associated with changes in the lipid-phase behaviour of thylakoid membranes. Photosynthetica 56, 254–264 (2018). https://doi.org/10.1007/s11099-018-0782-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-018-0782-z