Abstract
Purpose
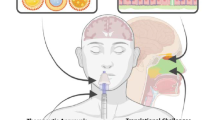
Intranasal administration enhances drug delivery to the brain by allowing targeted-drug delivery. Here, we investigated the properties that render a compound suitable for intranasal administration, and the differences between rodents and non-human primates in delivery to the brain.
Methods
The delivery of 10 low-permeable compounds to the brain, including substrates of efflux drug transporters expressed in the blood-brain barrier (didanosine, metformin, zolmitriptan, cimetidine, methotrexate, talinolol, ranitidine, atenolol, furosemide, and sulpiride) and two high-permeable compounds (ropinirole and midazolam) was evaluated following intranasal and intravenous administration in rats. Six of the 12 compounds (metformin, cimetidine, methotrexate, talinolol, sulpiride, and ropinirole) were also evaluated in monkeys, which have a similar nasal cavity anatomical structure to humans.
Results
In rats, most of the low-permeable compounds displayed an obvious increase in the brain/plasma concentration ratio (Kp) by intranasal administration (despite their substrate liability for efflux drug transporters); this was not observed with the high-permeable compounds. Similarly, intranasal administration increased Kp for all low-permeable compounds in monkeys.
Conclusions
Compound permeability is a key determinant of Kp increase by intranasal administration. This route of administration is more beneficial for low-permeable compounds and enhances their delivery to the brain in rodents and non-human primates.




Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the plasma concentration-time curve
- BBB:
-
Blood-brain barrier
- BCRP:
-
Breast cancer resistance protein
- CNS:
-
Central nervous system
- Kp :
-
Tissue/plasma concentration ratio
- LC/MS/MS:
-
Liquid chromatography/tandem mass spectrometry
- P-gp:
-
P-glycoprotein
References
Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52.
Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14.
Mittal D, Ali A, Md S, Baboota S, Sahni JK, Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014;21(2):75–86.
Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Chougule MB, Shoyele SA, et al. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release. 2018;281:139–77.
Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69(1):29–38.
Dhuria SV, Hanson LR, Frey WH 2nd. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–73.
Sakane T, Akizuki M, Taki Y, Yamashita S, Sezaki H, Nadai T. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the molecular weight of drugs. J Pharm Pharmacol. 1995;47(5):379–81.
Sakane T, Akizuki M, Yamashita S, Nadai T, Hashida M, Sezaki H. The transport of a drug to the cerebrospinal fluid directly from the nasal cavity: the relation to the lipophilicity of the drug. Chem Pharm Bull (Tokyo). 1991;39(9):2456–8.
Sakane T, Akizuki M, Yamashita S, Sezaki H, Nadai T. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the dissociation of the drug. J Pharm Pharmacol. 1994;46(5):378–9.
Genter MB, Krishan M, Augustine LM, Cherrington NJ. Drug transporter expression and localization in rat nasal respiratory and olfactory mucosa and olfactory bulb. Drug Metab Dispos. 2010;38(10):1644–7.
Thiebaud N, Menetrier F, Belloir C, Minn AL, Neiers F, Artur Y, et al. Expression and differential localization of xenobiotic transporters in the rat olfactory neuro-epithelium. Neurosci Lett. 2011;505(2):180–5.
Molinas A, Sicard G, Jakob I. Functional evidence of multidrug resistance transporters (MDR) in rodent olfactory epithelium. PLoS One. 2012;7(5):e36167.
Graff CL, Pollack GM. P-glycoprotein attenuates brain uptake of substrates after nasal instillation. Pharm Res. 2003;20(8):1225–30.
Qosa H, Miller DS, Pasinelli P, Trotti D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015;1628(Pt B):298–316.
Sakane T, Akizuki M, Yoshida M, Yamashita S, Nadai T, Hashida M, et al. Transport of cephalexin to the cerebrospinal fluid directly from the nasal cavity. J Pharm Pharmacol. 1991;43(6):449–51.
Westin UE, Bostrom E, Grasjo J, Hammarlund-Udenaes M, Bjork E. Direct nose-to-brain transfer of morphine after nasal administration to rats. Pharm Res. 2006;23(3):565–72.
Hanson LR, Roeytenberg A, Martinez PM, Coppes VG, Sweet DC, Rao RJ, et al. Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther. 2009;330(3):679–86.
Abd-Elal RM, Shamma RN, Rashed HM, Bendas ER. Trans-nasal zolmitriptan novasomes: in-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016;23(9):3374–86.
Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56(1):3–17.
Merkus FW, van den Berg MP. Can nasal drug delivery bypass the blood-brain barrier?: questioning the direct transport theory. Drugs R D. 2007;8(3):133–44.
Bitter C, Suter-Zimmermann K, Surber C. Nasal drug delivery in humans. Curr Probl Dermatol. 2011;40:20–35.
Gizurarson S. The relevance of nasal physiology to the design of drug absorption studies. Adv Drug Deliv Rev. 1993;11(3):329–47.
Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 2015;35(3):371–81.
Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Role of human placental apical membrane transporters in the efflux of glyburide, rosiglitazone, and metformin. Am J Obstet Gynecol. 2010;202(4):383 e1–7.
Yu L, Zeng S. Transport characteristics of zolmitriptan in a human intestinal epithelial cell line Caco-2. J Pharm Pharmacol. 2007;59(5):655–60.
Bicker J, Fortuna A, Alves G, Soares-da-Silva P, Falcao A. Elucidation of the impact of P-glycoprotein and breast Cancer resistance protein on the brain distribution of catechol-O-methyltransferase inhibitors. Drug Metab Dispos. 2017;45(12):1282–91.
Mao Q, Unadkat JD. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update. AAPS J. 2015;17(1):65–82.
Oswald S, Terhaag B, Siegmund W. In vivo probes of drug transport: commonly used probe drugs to assess function of intestinal P-glycoprotein (ABCB1) in humans. Handb Exp Pharmacol. 2011;201:403–47.
Lee K, Ng C, Brouwer KL, Thakker DR. Secretory transport of ranitidine and famotidine across Caco-2 cell monolayers. J Pharmacol Exp Ther. 2002;303(2):574–80.
Lin X, Skolnik S, Chen X, Wang J. Attenuation of intestinal absorption by major efflux transporters: quantitative tools and strategies using a Caco-2 model. Drug Metab Dispos. 2011;39(2):265–74.
Bai M, Ma Z, Sun D, Zheng C, Weng Y, Yang X, et al. Multiple drug transporters mediate the placental transport of sulpiride. Arch Toxicol. 2017;91:3873–84.
Kadono K, Akabane T, Tabata K, Gato K, Terashita S, Teramura T. Quantitative prediction of intestinal metabolism in humans from a simplified intestinal availability model and empirical scaling factor. Drug Metab Dispos. 2010;38(7):1230–7.
Kandimalla KK, Donovan MD. Localization and differential activity of P-glycoprotein in the bovine olfactory and nasal respiratory mucosae. Pharm Res. 2005;22(7):1121–8.
Shingaki T, Hidalgo IJ, Furubayashi T, Sakane T, Katsumi H, Yamamoto A, et al. Nasal delivery of P-gp substrates to the brain through the nose-brain pathway. Drug Metab Pharmacokinet. 2011;26(3):248–55.
Misra A, Kher G. Drug delivery systems from nose to brain. Curr Pharm Biotechnol. 2012;13(12):2355–79.
Ruigrok MJ, de Lange EC. Emerging insights for translational pharmacokinetic and pharmacokinetic-Pharmacodynamic studies: towards prediction of nose-to-brain transport in humans. AAPS J. 2015;17(3):493–505.
Nakamichi N, Kato Y. Physiological roles of carnitine/organic cation transporter OCTN1/SLC22A4 in neural cells. Biol Pharm Bull. 2017;40(8):1146–52.
Nakamichi N, Shima H, Asano S, Ishimoto T, Sugiura T, Matsubara K, et al. Involvement of carnitine/organic cation transporter OCTN1/SLC22A4 in gastrointestinal absorption of metformin. J Pharm Sci. 2013;102(9):3407–17.
Schriever VA, Reither N, Gerber J, Iannilli E, Hummel T. Olfactory bulb volume in smokers. Exp Brain Res. 2013;225(2):153–7.
Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847–55.
Yamada K, Hasegawa M, Kametani S, Ito S. Nose-to-brain delivery of TS-002, prostaglandin D2 analogue. J Drug Target. 2007;15(1):59–66.
Acknowledgement and Disclosures
All authors were employees of Takeda Pharmaceutical Company Limited when the study was performed. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Fig. 1
Time profiles of plasma concentration in rats after intravenous (open circle) or intranasal administration (closed square) (mean ± S.D., n = 3 / time point). (PNG 250 kb)
High resolution image
(EPS 93 kb)
Supplemental Fig. 2
Time profiles of olfactory bulb concentration in rats after intravenous (open circle) or intranasal administration (closed square) (mean ± S.D., n = 3 / time point). The concentration below the quantitation limit was assumed to be 0 and not shown in the figure. The bottom of the error bar, which extends to a negative y-axis value, is not shown. (PNG 254 kb)
High resolution image
(EPS 91 kb)
Supplemental Fig. 3
Time profiles of olfactory tract concentration in rats after intravenous (open circle) or intranasal administration (closed square) (mean ± S.D., n = 3 / time point). The concentration below the quantitation limit was assumed to be 0 and not shown in the figure. The bottom of the error bar, which extends to a negative y-axis value, is not shown. (PNG 248 kb)
High resolution image
(EPS 90 kb)
Supplemental Fig. 4
Time profiles of trigeminal nerve concentration in rats after intravenous (open circle) or intranasal administration (closed square) (mean ± S.D., n = 3 / time point). The bottom of the error bar, which extends to a negative y-axis value, is not shown. (PNG 255 kb)
High resolution image
(EPS 94 kb)
Supplemental Fig. 5
Time profiles of the rest of the brain concentration in rats after intravenous (open circle) or intranasal administration (closed square) (mean ± S.D., n = 3 / time point). The concentration below the quantitation limit was assumed to be 0 and not shown in the figure. The bottom of the error bar, which extends to a negative y-axis value, is not shown. (PNG 243 kb)
High resolution image
(EPS 90 kb)
Supplemental Fig. 6
The relationship of Kp,in/Kp,iv calculated by AUC and at 0.25 h in rat olfactory bulb (A), olfactory tract (B), trigeminal nerve (C), and rest of the brain (D). Solid line represents line of unity. The area between dashed lines represents an area within a 2-fold error. (PNG 96 kb)
High resolution image
(EPS 143 kb)
Rights and permissions
About this article
Cite this article
Iwasaki, S., Yamamoto, S., Sano, N. et al. Direct Drug Delivery of Low-Permeable Compounds to the Central Nervous System Via Intranasal Administration in Rats and Monkeys. Pharm Res 36, 76 (2019). https://doi.org/10.1007/s11095-019-2613-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2613-8