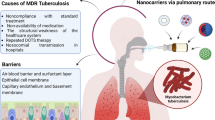
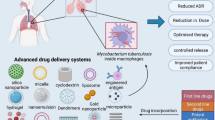
Abstract
Tuberculosis (TB) remains as the second most-deadly infection right behind the HIV/AIDS. Actually, in 2016, TB incidence was estimated in 10.4 million cases. Although an efficient and low-cost TB pharmacotherapy has been available for the last 50 years, the development of multi- and extra-drug-resistant Mycobacterium tuberculosis (Mtb) strains has put on the spot the necessity of improved TB regimens. In this framework, this review article presents the main relevant research outcomes of nanotechnology in TB. The novel delivery systems for antituberculosis drugs have been discussed. Moreover, the active-targeted nanomedicines to the Mtb reservoirs enlighten the possibility to eradicate low-replicant mycobacteria and diminish latent TB. Finally, we present an overview of the TB socio-economic impact and the cost-related features of TB regimens associated with the use of nanoformulations.




Similar content being viewed by others
Abbreviations
- AMs:
-
Alveolar macrophages
- FDCs:
-
Fixed dose combinations
- ETB:
-
Ethambutol
- INH:
-
Isoniazid
- LPs:
-
Liposomes
- MDR-TB:
-
Multi-drug resistant tuberculosis
- Mtb:
-
Mycobacterium tuberculosis
- NMs:
-
Niosomes
- NPs:
-
Nanoparticles
- PMs:
-
Polymeric micelles
- PYR:
-
Pyrazinamide
- RIF:
-
Rifampicin
- TB:
-
Tuberculosis
- XDR-TB:
-
Extra-drug resistant tuberculosis
References
World Health Organization. Global Tuberculosis Report 2017.; 2017. Available from: http://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf.
Peña DA, Rovetta AI, Hernández Del Pino RE, Amiano NO, Pasquinelli V, Pellegrini JM, et al. Mycobacterium tuberculosis dormancy antigen differentiates latently infected Bacillus Calmette-Guerin vaccinated individuals. EBioMedicine. 2015;2(8):882–8.
World Health Organization. WHO Global Tuberculosis Programme. TB: a global emergency, WHO report on the TB epidemic. Geneva: World Health Organization; 1994. Available from: http://www.who.int/iris/handle/10665/58749.
Porcel JM, Leung CC, Restrepo MI, Lee P. Year in review 2011: respiratory infections, tuberculosis, pleural diseases, bronchoscopic intervention and imaging. Respirology. 2012;17(3):573–82.
World Health Organization. Treatment of Tuberculosis. Guidelines for treatment of drug-susceptible tuberculosis and patient care 2017 Update; 2017. Available from: http://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf?sequence=1.
American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. Treatment of tuberculosis. Am J Respir Crit Care Med 2003; 167(4):603–662.
Stewart GRB, Young D. Tuberculosis: a problem with persistence. Nat Rev Microbiol. 2003;1(2):97–105.
Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis. 2003;188(8):1146–55.
Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1(1):20–30.
Riley RMW, Nyka N, Weintock P, Storey I, Sultan M, Wells W. Aerial dissemination of pulmonary tuberculosis: a two year study of contagion in a tuberculosis ward. Am J Hyg. 1959;142(1):185–96.
Fenton MJ, Vermeulen MW. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996;64(3):683–90.
Stenger S, Rollinghoff M. Role of cytokines in the innate immune response to intracellular pathogens. Ann Rheum Dis. 2001;60(3):43–6.
Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12(5):352–66.
Hashemian SM, Tabarsi P, Karam MB, Kahkouee S, Marjani M, Jamaati H, et al. Radiologic manifestations of pulmonary tuberculosis in patients of intensive care units. Int J Mycobacteriol. 2015;4(3):233–8.
Schnap**er D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, et al. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med. 2003;198(5):693–704.
Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb). 2004;84(1–2):93–101.
Tateosian NL, Pellegrini JM, Amiano NO, Rolandelli A, Casco N, Palmero DJ, et al. IL17A augments autophagy in Mycobacterium tuberculosis-infected monocytes from patients with active tuberculosis in association with the severity of the disease. Autophagy. 2017;13(7):1191–204.
Ivanyi J. Function and potentials of M. tuberculosis epitopes. Front Immunol. 2014;5:107.
Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. Madrid: Elsevier-Saunders Inc. 6th edition; 2008.
Garcia-Prats AJ, Willemse M, Seifart HI, Jordaan AM, Werely CJ, Donald PR, et al. Acquired drug resistance during inadequate therapy in a young child with tuberculosis. Pediatr Infect Dis J. 2014;33(8):883–5.
Panchagnula R, Agrawal S, Ashokraj Y, Varma M, Sateesh K, Bhardwaj V, et al. Fixed dose combinations for tuberculosis: lessons learned from clinical, formulation and regulatory perspective. Methods Find Exp Clin Pharmacol. 2004;26(9):703–72.
Preziosi P. Isoniazid: metabolic aspects and toxicological correlates. Curr Drug Metab. 2007;8(8):839–51.
Rivers EC, Mancera RL. New anti-tuberculosis drugs in clinical trials with novel mechanisms of action. Drug Discov Today. 2008;13(23–24):1090–8.
Moretton MA, Glisoni RJ, Chiappetta DA, Sosnik A. Molecular implications in the nanoencapsulation of the anti-tuberculosis drug rifampicin within flower-like polymeric micelles. Colloids Surf. B: Biointerfaces. 2010;79(2):467–79.
Gohel MC, Sarvaiya KG. A novel solid dosage form of rifampicin and isoniazid with improved functionality. AAPS PharmSciTech. 2007;8(3):E68.
El-Ridy MS, Mostafa DM, Shehab A, Nasr EA, Abd El-Alim S. Biological evaluation of pyrazinamide liposomes for treatment of Mycobacterium tuberculosis. Int J Pharm. 2007;330(1–2):82–8.
World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management, 2018. Available from: http://apps.who.int/iris/bitstream/handle/10665/260233/9789241550239-eng.pdf?sequence=1.
Du Toit LC, Pillay V, Danckwerts MP. Tuberculosis chemotherapy: current drug delivery approaches. Respir Res. 2006;7:118.
World Health Organization. Treatment of tuberculosis. Guidelines for national programmes. Geneva:World Health Organization, 2003. Available from: http://apps.who.int/iris/bitstream/handle/10665/67890/WHO_CDS_TB_2003.313_eng.pdf?sequence=1.
Chang K-C, Yew W-W. Management of difficult multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis: update 2012. Respirology. 2013;18(1):8–21.
Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers vol. 2 2016, Article number: 16076.
World Health Organization. Treatment guidelines for drug-resistant tuberculosis, 2016 update. Available from: http://apps.who.int/iris/bitstream/handle/10665/250125/9789241549639-eng.pdf;jsessionid=8E57A036E76B0B028129B6AC6678C423?sequence=1.
Caminero JA, Scardigli A. Classification of antituberculosis drugs: a new proposal based on the most recent evidence. Eur Respir J. 2015;46(4):887–93.
Falzon D, Jaramillo E, Schünemann HJ, Arentz M, Bauer M, Bayona J, et al. WHO guidelines for the programmatic management of drug resistant tuberculosis: 2011 update. Eur Respir J. 2011;38(3):516–28.
Tiberi S, Scardigli A, Centis R, D'Ambrosio L, Muñoz-Torrico M, Salazar-Lezama MÁ, et al. Classifying new anti-TB drugs: rationale and future perspectives. Int J Infect Dis. 2017;56:181–4.
World Health Organization. The use of delamanid in the treatment of multidrug-resistant tuberculosis. Interim policy guidance. WHO/HTM/TB2014.23. Geneva: World Health Organization, 2014. Available from: http://apps.who.int/iris/bitstream/handle/10665/137334/WHO_HTM_TB_2014.23_eng.pdf?sequence=1.
Harausz EP, Garcia-Prats AJ, Seddon JA, Schaaf HS, Hesseling AC, Achar J, et al. On behalf of the sentinel project on pediatric drug-resistant tuberculosis. New drugs, repurposed drugs, and novel regimens for children with multidrug-resistant tuberculosis: practice-based recommendations. Am J Respir Crit Care Med. 2016;195(10):1–58.
Gler MT, Skripconoka V, Sanchez-Garavito E, **ao H, Cabrera-Rivero JL, Vargas-Vasquez DE, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366(23):2151–60.
Tadolini M, Lingtsang RD, Tiberi S, Enwerem M, D'Ambrosio L, Sadutshang TD, et al. First case of extensively drug-resistant tuberculosis treated with both delamanid and bedaquiline. Eur Respir J. 2016;48(3):935–8.
Wallis RS. Cardiac safety of extensively drug-resistant tuberculosis regimens including bedaquiline, delamanid and clofazimine. Eur Respir J. 2016;48(5):1526–7.
Laghari M, Darwis Y, Memon AH, Khan AA, Abdulbaqi IMT, Assi RA. Nanoformulations and clinical trial candidates as probably effective and safe therapy for tuberculosis. Trop J Pharm Res. 2016;15(1):201–11.
Bawarski WE, Chidlowsky E, Bharali DJ, Mousa SA. Emerging nanopharmaceuticals. Nanomedicine. 2008;4(4):273–82.
Abed N, Couvreur P. Nanocarriers for antibiotics: a promising solution to treat intracellular bacterial infections. Int J Antimicrob Agents. 2014;43(6):485–96.
Changsan N, Chan HK, Separovic F, Srichana T. Physicochemical characterization and stability of rifampicin liposome dry powder formulations for inhalation. J Pharm Sci. 2009;98(2):628–39.
Manca ML, Sinico C, Maccioni AM, Diez O, Fadda AM, Manconi M. Composition influence on pulmonary delivery of rifampicin liposomes. Pharmaceutics. 2012;4(4):590–606.
Chimote G, Banerjee R. In vitro evaluation of inhalable isoniazid-loaded surfactant liposomes as an adjunct therapy in pulmonary tuberculosis. J Biomed Mater Res B Appl Biomater. 2010;94(1):1–10.
Booysen LL, Kalombo L, Brooks E, Hansen R, Gilliland J, Gruppo V, et al. In vivo/in vitro pharmacokinetic and pharmacodynamic study of spray-dried poly-(dl-lactic-co-glycolic) acid nanoparticles encapsulating rifampicin and isoniazid. Int J Pharm. 2013;444(1–2):10–7.
Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18(2):113–20.
Pooja D, Tunki L, Kulhari H, Reddy BB, Sistla R. Characterization, biorecognitive activity and stability of WGA grafted lipid nanostructures for the controlled delivery of rifampicin. Chem Phys Lipids. 2015;193:11–7.
Singh H, Bhandari R, Kaur IP. Encapsulation of rifampicin in a solid lipid nanoparticulate system to limit its degradation and interaction with isoniazid at acidic pH. Int J Pharm. 2013;446(1–2):106–11.
Singh H, **dal S. Singh, Sharma G, Kaur IP. Nano-formulation of rifampicin with enhanced bioavailability: development, characterization and in-vivo safety. Int J Pharm. 2015;485(1–2):138–51.
Vieira ACC, Chaves LL, Pinheiro S, Pinto S, Pinheiro M, Lima SC, et al. Mucoadhesive chitosan-coated solid lipid nanoparticles for better management of tuberculosis. Int J Pharm. 2018;536(1):478–85.
Banik N, Hussain A, Ramteke A, Sharma HK, Maji TK. Preparation and evaluation of the effect of particle size on the properties of chitosan-montmorillonite nanoparticles loaded with isoniazid. RSC Adv. 2012;2:10519–28.
Trousil J, Filippov SK, Hrubý M, Mazel T, Syrová Z, Cmarko D, et al. System with embedded drug release and nanoparticle degradation sensor showing efficient rifampicin delivery into macrophages. Nanomedicine. 2017;13(1):307–15.
Gajendiran M, Gopi V, Elangovan V, Murali RV, Balasubramanian S. Isoniazid loaded core shell nanoparticles derived from PLGA-PEG-PLGA tri-block copolymers: in vitro and in vivo drug release. Colloids Surf B: Biointerfaces. 2013;104:107–15.
Pandey R, Khuller GK. Nanoparticle-based oral drug delivery system for an injectable antibiotic - streptomycin. Evaluation in a murine tuberculosis model. Chemotherapy. 2007;53(6):437–41.
Pandey R, Sharma A, Zahoor A, Sharma S, Khuller GK, Prasad B. Poly (DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J Antimicrob Chemother. 2003;52(6):981–6.
Ahmad Z, Sharma S, Khuller GK. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int J Antimicrob Agents. 2005;26(4):298–303.
Cagel M, Tesan FC, Bernabeu E, Salgueiro MJ, Zubillaga MB, Moretton MA, et al. Polymeric mixed micelles as nanomedicines: achievements and perspectives. Eur J Pharm Biopharm. 2017;113:211–28.
Owen SC, Chan DPY, Shoichet MS. Polymeric micelle stability. Nano Today. 2012;7:53–65.
Moretton MA, Höcht C, Taira C, Sosnik A. Rifampicin-loaded ‘flower-like’ polymeric micelles for enhanced oral bioavailability in an extemporaneous liquid fixed-dose combination with isoniazid. Nanomedicine (London). 2014;9(11):1635–50.
Moretton MA, Chiappetta DA, Sosnik A. Cryoprotection-lyophilization and physical stabilization of rifampicin-loaded flower-like polymeric micelles. J R Soc Interface. 2012;9(68):487–502.
Grotz E, Bernabeu E, Pappalardo M, Chiappetta DA, Moretton MA. Nanoscale Kolliphor® HS 15 micelles to minimize rifampicin selfaggregation in aqueous media. J Drug Deliv Sci Technol. 2017;41:1–6.
Praphakar RA, Munusamy MA, Rajan M. Development of extended-voyaging anti-oxidant linked amphiphilic polymeric Nanomicelles for anti-tuberculosis drug delivery. Int J Pharm. 2017;524(1–2):168–77.
Upadhyay S, Khan I, Gothwal A, Pachouri PK, Bhaskar N, Gupta UD. Conjugated and entrapped HPMA-PLA Nano-polymeric micelles based dual delivery of first line anti TB drugs: improved and safe drug delivery against sensitive and resistant Mycobacterium tuberculosis. Pharm Res. 2017;34(9):1944–55.
Jain JP, Ayen WY, Kumar N. Self assembling polymers as Polymersomes for drug delivery. Curr Pharm Des. 2011;17(1):65–79.
Chang H-Y, Sheng Y-J, Tsao H-K. Structural and mechanical characteristics of Polymersomes. Soft Matter. 2014;10(34):6373–681.
Moretton MA, Cagel M, Bernabeu E, Gonzalez L, Chiappetta DA. Nanopolymersomes as potential carriers for rifampicin pulmonary delivery. Colloids Surf B: Biointerfaces. 2015;136:1017–25.
Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release. 2014;185(1):22–36.
Junyaprasert VP, Teeranachaideekul V, Supaperm T. Effect of charged and non-ionic membrane additives on physicochemical properties and stability of niosomes. AAPS PharmSciTech. 2008;9(3):851–9.
El-Ridy MS, Abdelbary A, Nasr EA, Khalil RM, Mostafa DM, El-Batal AI, et al. Niosomal encapsulation of the antitubercular drug, pyrazinamide. Drug Dev Ind Pharm. 2011;37(9):1110–8.
El-Ridy MS, Yehia SA, Kassem MA, Mostafa DM, Nasr EA, Asfour MH. Niosomal encapsulation of ethambutol hydrochloride for increasing its efficacy and safety. Drug Delivery. 2015;22(1):21–36.
Mehta SK, **dal N, Kaur G. Quantitative investigation, stability and in vitro release studies of anti-TB drugs in triton niosomes. Colloids Surf B: Biointerfaces. 2011;87(1):173–9.
Mehta SK, **dal N. Formulation of Tyloxapol niosomes for encapsulation, stabilization and dissolution of anti-tubercular drugs. Colloids Surf B: Biointerfaces. 2013;101:434–41.
World Health Organization. Frequently asked questions about the implementation of the new WHO recommendation on the use of the shorter MDR-TB regimen under programmatic conditions 2016. Available from: http://www.who.int/tb/areas-of-work/drug-resistant tb/treatment/FAQshorter_MDR_regimen.pdf.
Adikwu E, Deo O. Fluoroquinolones reported hepatotoxicity. Pharmacology and Pharmacy. 2012;3:328–36.
Mustafa S, Devi VK, Pai RS. Effect of PEG and water-soluble chitosan coating on moxifloxacin-loaded PLGA long-circulating nanoparticles. Drug Deliv Transl Res. 2016;7(1):27–36.
Sarfraz M, Shi W, Gao Y, Clas SD, Roa W, Bou-Chacra N, et al. Immune response to antituberculosis drug-loaded gelatin and polyisobutyl-cyanoacrylate nanoparticles in macrophages. Ther Deliv. 2016;7(4):213–28.
Bhardwaj A, Mehta S, Yadav S, Singh SK, Grobler A, Goyal AK, et al. Pulmonary delivery of antitubercular drugs using spray-dried lipid – polymer hybrid nanoparticles. Artif Cells Nanomed Biotechnol. 2015;44(6):1544–55.
Adams LB, Sinha I, Franzblau SG, Krahenbuhl JL, Mehta RT. Effective treatment of acute and chronic murine tuberculosis with liposome-encapsulated Clofazimine. Antimicrob Agents Chemother. 1999;43(7):1638–43.
Gaidukevich SK, Mikulovich YL, Smirnova TG, Andreevskaya SN, Sorokoumova GM, Chernousova LN, et al. Antibacterial effects of liposomes containing phospholipid Cardiolipin and fluoroquinolone Levofl oxacin on Mycobacterium tuberculosis with extensive drug resistance. Bull Exp Biol Med. 2016;160(5):675–6.
Breen RA, Swaden L, Ballinger J, Lipman MC. Tuberculosis and HIV co-infection: a practical therapeutic approach. Drugs. 2006;66(18):2299–308.
Centers for Disease Control and Prevention. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis 2018. Available from: https://www.cdc.gov/tb/publications/guidelines/tb_hiv_drugs/recommendations03.htm.
Gaspar MM, Cruz A, Penha AF, Reymăo J, Sousa AC, Eleutério CV, et al. Rifabutin encapsulated in liposomes exhibits increased therapeutic activity in a model of disseminated tuberculosis. Int J Antimicrob Agents. 2008;31(1):37–45.
World Health Organization. The end TB Strategy 2015. Available from: http://www.who.int/tb/End_TB_brochure.pdf?ua=1.
World Health Organization. Interim guidance on the use of bedaquiline to treat MDR-TB 2018. Available from: http://www.who.int/tb/challenges/mdr/bedaquiline/en/.
Field SK. Bedaquiline for the treatment of multidrug-resistant tuberculosis: great promise or disappointment? Ther Adv Chronic Dis. 2015;6(4):170–84.
An Open-label RCT to Evaluate a New Treatment Regimen for Patients With Multi-drug Resistant Tuberculosis (NEXT); 2015. Identifier NCT02454205. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02454205?term=bedaquiline&rank=28.
The Evaluation of a Standard Treatment Regimen of Anti-tuberculosis Drugs for Patients With MDR-TB (STREAM); 2015. Identifier NCT02409290. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02409290?term=stream&rank=8.
Safety and Efficacy of Various Doses and Treatment Durations of Linezolid Plus Bedaquiline and Pretomanid in Participants With Pulmonary TB, XDR-TB, Pre- XDR-TB or Non-responsive/Intolerant MDR-TB (ZeNiX); 2017. Identifier NCT03086486. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03086486?term=bedaquiline&rank=15.
A Phase 2 Open Label Partially Randomized Trial to Evaluate the Efficacy, Safety and Tolerability of Combinations of Bedaquiline, Moxifloxacin, PA-824 and Pyrazinamide in Adult Subjects With Drug-Sensitive or Multi Drug-Resistant Pulmonary Tuberculosis. (NC-005); 2014. Identifier NCT02193776. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02193776?term=bedaquiline&rank=14.
World Health Organization. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis 2013. Available from: http://apps.who.int/iris/bitstream/10665/84879/1/9789241505482_eng.pdf.
Ritsema JAS, Herschberg EMA, Borgos SE, Løvmo C, Schmid, RYM, te Welscher, YM, et al. Relationship between polarities of antibiotic and polymer matrix on nanoparticle formulations based on aliphatic polyesters. Int J Pharm 2017. Article in press https://doi.org/10.1016/j.ijpharm.2017.11.017.
De Matteis L, Jary D, Lucía A, García-Embid S, Serrano-Sevilla I, Pérez D, et al. New active formulations against M. tuberculosis: Bedaquiline encapsulation in lipid nanoparticles and chitosan nanocapsules. Chem Eng J. 2018;340:181–91.
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25.
Traini D, Young PM. Drug delivery for tuberculosis: is inhaled therapy the key to success? Ther Deliv. 2017;8(10):819–21.
Irache JM, Salman HH, Gamazo G, Espuelas S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin Drug Deliv. 2008;5(6):703–24.
Song X, Lin Q, Guo L, Fu Y, Han J, Ke H, et al. Rifampicin loaded Mannosylated cationic nanostructured lipid carriers for alveolar macrophage-specific delivery. Pharm Res. 2015;32(5):1741–51.
Vieira ACC, Chaves LL, Pinheiro M, Costa Lima SA, Ferreira D, Bruno Sarmento B, et al. Mannosylated solid lipid nanoparticles for the selective delivery of rifampicin to macrophages. Artif Cells Nanomed Biotechnol. 2018;12:1–11.
Maretti E, Costantino L, Rustichelli C, Leo E, Croce MA, Buttini F, et al. Surface engineering of solid lipid nanoparticle assemblies by methyl α-D-mannopyranoside for the active targeting to macrophages in anti-tuberculosis inhalation therapy. Int J Pharm. 2017;528(1–2):440–51.
Costa A, Sarmento B, Seabra V. Mannose-functionalized solid lipid nanoparticles are effective in targeting alveolar macrophages. Eur J Pharm Sci. 2018;114:103–13.
Moretton MA, Chiappetta DA, Andrade F. das Neves J, Ferreira D, Sarmento B, et al. hydrolyzed galactomannan-modified nanoparticles and flower-like polymeric micelles for the active targeting of rifampicin to macrophages. J Biomed Nanotechnol. 2013;9(6):1076–87.
Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6(3):139–50.
Kumar PV, Asthana A, Dutta T, Jain NK. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J Drug Target. 2006;14(8):546–56.
Katoh S, Miyagi T, Taniguchi H, Matsubara Y-i, Kadota J-i, Tominaga A, et al. Cutting edge: an inducible Sialidase regulates the hyaluronic acid binding ability of CD44-bearing human monocytes. J Immunol. 1999;162(9):5058–61.
Gao Y, Sarfraz MK, Clas SD, Roa W, Löbenberg R. Hyaluronic acid-tocopherol succinate-based self-assembling micelles for targeted delivery of rifampicin to alveolar macrophages. J Biomed Nanotechnol. 2015;11(8):1312–29.
World Health Organization. Global TB Report. 2015. Available from: http://www.who.int/iris/handle/10665/191102. Accessed Sept 2018.
Zwerling A, Dowdy D, von Delft A, Taylor H, Merritt MW. Incorporating social justice and stigma in cost-effectiveness analysis: drug-resistant tuberculosis treatment. Int J Tuberc Lung Dis. 2017;21(11):69–74.
Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19(3):311–30.
Patil K, Bagade S, Bonde S, Sharma S, GauravSaraogi G. Recent therapeutic approaches for the management of tuberculosis: challenges and opportunities. Biomed Pharmacother. 2018;99:735–45.
Mohammad IS, He W, Yin L. Understanding of human ATP binding cassette superfamily and novel multidrug resistance modulators to overcome MDR. Biomed Pharmacother. 2018;100:335–48.
te Brake LHM, de Knegt GJ, de Steenwinkel JE, van Dam TJP, Burger DM, Russel FGM, et al. The role of efflux pumps in tuberculosis treatment and their promise as a target in drug development: unraveling the black box. Annu Rev Pharmacol Toxicol. 2018;58:271–91.
Li P, Gu Y, Li J, **e L, Li X, **e J. Mycobacterium tuberculosis major facilitator superfamily transporters. J Membr Biol. 2017;250(6):573–85.
Hamed K, Debonnett L. Tobramycin inhalation powder for the treatment of pulmonary Pseudomonas aeruginosa infection in patients with cystic fibrosis: a review based on clinical evidence. Ther Adv Respir Dis. 2017;11(5):193–209.
Manconi M, Manca ML, Valentia D, Escribano E, Hillaireau H, Anna Maria Fadda AM, et al. Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. Int J Pharm. 2017;525(1):203–10.
Wang L, Zhou Y, Wu M, Wu M, Li X, Gong X, et al. Functional nanocarrier for drug and gene delivery via local administration in mucosal tisúes. Nanomedicine (London). 2018;13(1):69–88.
Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respir Care. 2005;50(9):1209–27.
Abdelwahed W, Degobert G. Fessi H. investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur J Pharm Biopharm. 2006;63(2):87–94.
Igarashi M, Ishizaki Y, Takahashi Y. New antituberculous drugs derived from natural products: current perspectives and issues in antituberculous drug development. J Antibiot. 2018;71(1):15–25.
Acknowledgments and Disclosures
Authors thank the Universidad de Buenos Aires (Grant UBACyT 20020130200038BA). Estefania Grotz and Maximiliano Cagel are supported by doctoral scholarship of CONICET. Marcela A. Moretton, Nancy Tateosian, Nicolas Amiano, Ezequiel Bernabeu, and Diego A. Chiappetta are partially supported by CONICET, Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grotz, E., Tateosian, N., Amiano, N. et al. Nanotechnology in Tuberculosis: State of the Art and the Challenges Ahead. Pharm Res 35, 213 (2018). https://doi.org/10.1007/s11095-018-2497-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2497-z