A procedure for the determination of oseltamivir in human plasma by high-performance liquid chromatography( tandem mass spectrometry (HPLC-MS/MS) was proposed and validated. Arapid and easy-to-use method of liquid(liquid extraction with ethyl acetate using venlafaxine as an internal standard was used during sample preparation. The addition of benzoic acid to aqueous acetonitrile solutions of the analyte was shown to prevent its oxidative degradation. The detection limit and limit of quantitation were 0.08 and 0.30 ng/mL, respectively; the calibration range, 0.3-200 ng/mL (R2 = 0.9937); the total analysis time, 3.2 min. The within- and between-run accuracy ranged from 97 to 105%. The precision was <10%. The proposed procedure was characterized by selective determination of the analyte, the absence of significant matrix effects, the ability to dilute samples with high analyte concentrations, and satisfactory extraction recovery (≥89%). The analyte was stable when stored in plasma samples (4 h at room temperature, 31 d at (80°C, after three freeze(thaw cycles) and extracts under autosampler storage conditions (24 h at 15°C). The procedure was successfully used for oseltamivir quantitation in actual plasma samples from healthy volunteers obtained during a bioequivalence study of the new generic drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
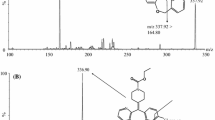
Oseltamivir [(3R,4R,5S)-4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-1-cyclohexen-1-carboxylic acid ethyl ester, OST, Fig. 1] is a prodrug that is rapidly hydrolyzed in vivo by carboxylesterase to form the active metabolite oseltamivir carboxylate [1]. In turn, the latter selectively inhibits neuraminidase of influenza A and B viruses [1, 2]. The drug is used in medical practice as the salt oseltamivir phosphate [1]. Nevertheless, drugs containing OST as salts with various organic acids are being widely developed [3].
It has now been reported that OST is being used to treat immune thrombocytopenia [4] and several cardiovascular diseases [5]. The synthesis of a conjugate of OST and bovine serum albumin that acted as a promising multivalent inhibitor of influenza neuraminidase and had antiviral activity superior to that of starting OST azidohexyl ester has been described [6]. A formulation of powder containing an inclusion complex of OST with hydroxypropyl-β-cyclodextrin was developed to produce a stable aqueous suspension of the drug [2]. Use of combination therapy of OST and baloxavir was shown to decrease the selection pressure for viruses with reduced drug susceptibility [7]. This expanded the arsenal of already existing methods for overcoming viral resistance to antiviral drugs (mutation H275Y responsible for drug resistance to OST appeared only for monotherapy). The combination of OST with hydroxychloroquine turned out to be more effective as antiviral therapy with respect to the duration of treatment of COVID-19 patients [8]. However, numerous deleterious necrotic and fibrotic histopathological changes in liver and kidneys, increased serum renal and hepatic toxicity markers, and reduced levels of serum total protein, albumin, and K+ ions were observed with therapeutic (twice for 5 d) and long-term (once for 45 d) peroral administration of OST to rats at a dose of 6.75 mg/kg [9]. Other researchers concluded based on their own research results that OST treatment did not substantially shorten the duration of fever and did not alleviate the disease symptoms in children with influenza infections [10].
Several recent studies (2012 – 2016) [1, 11,12,13] described bioanalytical methods for determining OST in human blood plasma using liquid chromatography(tandem mass spectrometry (HPLC-MS/MS) that also included earlier studies on the topic. The lower limit of quantitation (LLOQ) varied from 0.34 to 3 ng/mL; the volume of the injected aliquot was 5 [13] or 10 μL [1, 11, 12]. The internal standard (IS) was the deuterated analog of OST [11, 12] or ambroxol [13]. Biological samples were prepared by solid-phase extraction [1] or protein precipitation [11,12,13]. The degree of OST extraction in all instances was >90% (the numerical value of the degree of extraction was not found by us in one instance [12]). The average retention time of the analyte (total analysis time given in parentheses) was 5.00 (9.0) [11], 1.49 (4.5) [12], or 1.56 (2.0) min [1] (the required information was missing in one instance [13]).
The aim of the present work was to develop and validate an analytical technique for determining OST in human blood plasma using HPLC-MS/MS with liquid(liquid extraction as the main sample-preparation method. Special attention was paid to matrix effects, analysis time, and OST stability in the biological matrix and aqueous acetonitrile solution.
Experimental Part
Reference standards of oseltamivir phosphate (Active Pharma Sciences Ltd., India) and venlafaxine hydrochloride (Aarti Industries Ltd., India), which acted as an IS (Fig. 1), of 99.4% and 99.0% purity, respectively; acetonitrile (MeCN, type 0, high purity; Kriokhrom, Russia); ethyl acetate (EtOAc, high purity; EKOS-1, Russia); formic acid (98.0 – 100%; Sigma-Aldrich, USA), ammonium formate and acetate (≥99.0%, Sigma-Aldrich, USA); ammonia (high purity, Sigma Tek, Russia); ammonium sulfate (high purity, Khimreaktivsnab, Russia); and benzoic acid (high purity, Component- Reaktiv, Russia) were used in the work. Ultrapure H2O (type I) was obtained using a Millipore Direct-Q 5 UV specialized water-purification system (Merck, USA). Samples of intact healthy volunteer plasma (dipotassium EDTA anticoagulant) were acquired from a private organization and stored at (32°C.
Analytical HPLC-MS/MS included a DGU-20A5R degasser, LC-20ADXR high-pressure pump, SIL-20ACXR autosampler, STO-20A thermostat (Shimadzu, Japan), Triple Quad 3500 tandem mass spectrometer with a triple quadrupole (AB Sciex, USA), Luna C18(2) chromatography column (100 × 4.6 mm, 5 μm, 100 Å; Phenomenex, USA) with a precolumn cartridge, and a Genius N2 gas generator (Peak Scientific, Great Britain).
A Microlab® STARlet automated liquid handler (Hamilton, Switzerland), MSV-3500 multi speed tube vortex (Biosan, Latvia), Rotator Multi RS-60 (Biosan, Latvia), and SL 16R refrigerated centrifuge (Thermo Scientific, USA) were used for sample preparation.
Analyst 1.7.2 and MultiQuant 3.0.3 (AB Sciex, USA) software was used to collect and process HPLC-MS/MS data, respectively.
Standard solutions. Stock solutions of OST and IS (c = 1000 μg/mL) were prepared by dissolving accurate weights in MeCN:H2O (7:3, v/v). The OST solution also contained benzoic acid (BA, c = 200 μg/mL). Then, working solutions (caddit BA = 100 μg/mL, each solution) of concentrations 40, 4, 0.2, and 0.1 μg/mL (OST) and 2, 0.1, and 0.025 μg/mL (IS) were prepared from the stock solutions. In turn, calibration solutions of OST (n = 9) in the concentration range 6 – 4000 ng/mL; quality control solutions with OST concentrations 18, 600, and 3600 ng/mL; and solutions used for validation of the “dilution by 2 and 4 times” parameter (7200 and 14,400 ng/mL, respectively; BA additive = 200 μg/mL, each solution, MeCN:H2O solvent) were prepared. Separate working solutions were used to prepare the calibration solutions and the quality control solutions.
All stock and working solutions were stored in vessels of dark glass in freezers at (32°C. Calibration solutions, quality control solutions, and solutions for validating the “dilution” parameter were stored at 4°C in vials of transparent glass.
Calibration, control, null, and blank solutions. Intact human plasma samples were thawed at room temperature before analysis. Then, an aliquot (285 μL) was placed into an Eppendorf tube and treated with calibration standard or control solution (15 μL). Null and blank samples were prepared by adding to the plasma MeCN:H2O mixture (15 μL). The OST concentration range in the calibration solutions was 0.3 – 200 ng/mL (n = 9). The concentrations in quality control samples (QCS) were 0.3 ng/mL (LLOQ), 0.9 ng/mL (QCS1), 30 ng/mL (QCS2), and 180 ng/mL (QCS3). The OST concentrations in the samples used to validate the “dilution by 2 and 4 times” parameter were 360 and 720 ng/mL, respectively.
Sample preparation. Calibration, control, null, and actual samples were treated with IS (60 μL, c = 0.025 μg/mL), aqueous NH4OH solution (30 μL, 12.5%, v/v), ammonium sulfate solution (200 μL, 3 M), and EtOAc (800 μL, 60 μL of MeCN:H2O mixture instead of IS to blank solutions). Then, the mixture was stirred on the tube vortex (2100 rpm, 8 min) and centrifuged (12,000 g, 6 min, 4°C). The upper organic fraction (500 μL) was collected and transferred to a vial of colorless glass for further analysis.
HPLC-MS/MS analytical conditions. Elution used isocratic mode and a mobile phase of MeCN (eluent B) and a mixture of HCOOH (0.2 vol%), HCOONH4 (5 mM), and MeCN (1 vol%) in H2O (eluent A) in a 90:10 ratio (v/v), respectively. The volume of injected aliquot was 2 μL. The eluent flow rate was 0.60 mL/min. The autosampler and column temperatures were 15 and 40°C, respectively. The average retention time of OST was 2.32 ± 0.02 min; of IS, 2.56 ± 0.03 min. The total analysis time was 3.20 min (the waste valve was open until 1.80 min).
Analytes were detected using tandem mass spectrometry with electrospray ionization in selected reaction monitoring mode (recording positive ions) of the transitions (m/z) 313.3 → 166.1 (OST) and 278.1 → 58.1 (IS). The signal accumulation time was 150 ms. Table 1 presents the MS/MS detection conditions that were optimized beforehand to ensure the maximum instrumental sensitivity by direct syringe injection of analyte solution into the ion source or flow-injection with injection of aliquots through the chromatography system.
The analysis method was validated according to recommendations of the SP RF, XIVth Ed. [14] and the European Medicines Agency [15] and included the parameters selectivity, lower limit of quantitation, linearity, within- and between-run accuracy and precision, carryover effect, matrix effect, dilution, and analyte stability in the biological matrix and aqueous organic solvent.
The analytical method was tested on an actual sample using a comparative pharmacokinetic study of the bioequivalence of two medicines (Tamiflu®, F. Hoffmann-La Roche Ltd., Switzerland and Oseltamivir, OOO Velfarm, Bright Way Group, Russia) containing oseltamivir as the active ingredient (capsules, 75 mg, peroral, on fasting) in healthy volunteers of both sexes from 18 to 45 years old (n = 46).
Blood (6 mL) was collected in containers containing NaF and potassium oxalate before and 0.167, 0.333, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.5, 3.0, 3.5, 4.0, 6.0, 8.0, 10.0, 12.0, and 24.0 h after administration of the drug. Then, the samples were centrifuged (3000 g, 10 min, 4°C). The obtained plasma was collected and transferred to polypropylene cryotubes that were stored at (80°C until the start of analysis. Samples were thawed at room temperature before analysis, stirred on a multi rotor (3 min), and centrifuged (3000 g, 3 min, 20°C). An aliquot (300 μL) was taken for further sample preparation.
Results and Discussion
Development of HPLC-MS/MS analytical method. Several chromatography columns were initially examined. They contained C18(2) or C18 sorbent and had different lengths and diameters (100 × 4.6 and 50 × 2.0 mm) and sorbent particle (3 – 5 μm) and pore sizes (80 and 100 Å). As a result, the C18(2) column (100 _ 4.6 mm, 5 μm, 100 Å) was selected because the analyte peak was strongest although asymmetric (AS = 1.81 ± 0.23) if it was used. Next, various modifiers to eluent A (H2O) were tested, i.e., 0.1% HCOOH, a mixture of 0.1% HCOOH and 5 mM HCOONH4, 5 and 10 mM HCOONH4, and 5 mM CH3COONH4. The strongest analyte intensity was observed by using 0.1% HCOOH or its mixture with 5 mM HCOONH4. In the latter case, the peak symmetry was also satisfactory (AS = 1.39 ± 0.12). The level of background noise in the chromatograms increased and the analyte signal dropped if the HCOONH4 concentration was increased from 5 to 10 mM in the tested mixture. However, the analyte response significantly increased if the HCOOH content was increased from 0.1 to 0.2%. Thus, a mixture of 0.2% HCOOH and 5 mM HCOONH4 with 1% MeCN was selected as eluent A. The signal and shape of the analyte peak was not affected by adding HCOOH to eluent B (MeCN). Other researchers used 0.1% HCOOH [11, 13], 2.5 mM HCOOH [12], or 10 mM HCOONH4 [1] as additives to eluent A.
A full factor experiment was used to select the eluent flow rate (mL/min, x1) and content of organic phase (vol%, x2) in the composition. The following parameters were chosen as the plan center: 0.55 mL/min with eluent B content 85 vol% (variation interval ±0.05 mL/min and eluent B content ±5%). The unknown variable (y) was the analyte response as the ratio of its signal to the background noise. Table 2 presents the experiment planning matrix after normalization of the factors (y = 351 in the center of the plan). This produced a linear model of the process in the vicinity of point (0.55, 85) as \(y=298.75-3.25{\overline{x} }_{1}+42.75{\overline{x} }_{2}-32.25{\overline{x} }_{1}{\overline{x} }_{2}\), from which it followed that the system response should be greater if the eluent flow rate was decreased and the volume fraction of the organic phase in it was increased. The following parameters were chosen as the final version considering the analyte retention time: flow rate 0.60 mL/min and eluent B 90 vol%.
Analyte stability in aqueous MeCN solutions. Special attention was paid before to the analyte stability not only in the biological matrix but also in aqueous media because OST is susceptible to oxidation to oseltamivir carboxylate during storage [1]. Therefore, an MeCN solution of dichlorvos (esterase inhibitor) was used as a stabilizer. BAwas proposed as such an additive in the present work.
An analysis of the calibration curves showed that the analyte was destroyed already after 1 d without added stabilizer (Fig. 2). However, satisfactory agreement of the calibration curves among themselves was observed if BA was added and indicated that the analyte was stable for a minimum of one month (Fig. 2). It is noteworthy that addition of small amounts (5 and 15 μL) of BA solution (c = 1 mg/mL) to the calibration solutions did not prevent oxidation of OST.
Calibration curves for oseltamivir in aqueous acetonitrile solution without added benzoic acid (a): freshly prepared calibration solutions (1 ), after 2 d (2 ), after 5 d (3 ), after 6 d (4 ); and with added benzoic acid (b ): after 26 d (1 ), freshly prepared calibration solutions (2 ), after 1, 3, 5, and 22 d (3 – 6 ).
Extraction of analyte and IS from biological matrix. As mentioned before, solid phase extraction or protein precipitation was used before to extract OST [1, 11,12,13]. Liquid( liquid extraction was proposed as the principal sample-preparation method in the present work. Initially, the extractant was EtOAc (800 μL) with various additives, e.g., 30 μL of 12.5 vol% NH3 (a), 50 μL of 12.5 vol% NH3(b), 30 μL of 12.5 vol% NH3 + 200 μL of 3M (NH4)2SO4 (c), 200 μL of 3 M (NH4)2 SO4 (d), and without additives (e). The degree of extraction (R) of the analyte and IS was estimated using QCS1 and QCS3 (n = 6 for each level) and the ratio R (%) = S1/S2 ·100%, where S1 is the peak area of the sample from the sample preparation procedure and S2 , the peak area of the sample representing 100% extraction efficiency. The average degree of analyte extraction varied from 78 to 94% for QCS1 or from 74 to 89% for QCS3 if the additives given in points a-c were used. The IS in all three instances had R ≈ 81%. The quantity R = 34% (QCS1) and 1% (QCS3) for the analyte and R ≈ 13% for the IS if only (NH4)2 SO4 (point d) was used as the additive. The analyte was detected only in trace quantities (signal/noise ratio for the analyte was <3.0) and R ≈ 8% for the IS without any additives (point e). The results indicated that the alkalinity created by adding ammonia solution had an important role in extracting the analyte and IS. This also followed from the chemical structures of the tested molecules (Fig. 1) because the amine N atom in their structures determined the basic properties of the compounds. Thus, addition of ammonia prevented protonation of the N atom and conversion of the analyte and IS to the neutral species, which were then successfully extracted into the organic solvent.
Other extractants were not examined considering the satisfactory results for the degree of extraction of the analyte and IS by EtOAc with added ammonia and ammonium sulfate (point c; 94 ± 8% for QCS1 and 89 ± 8% for QCS3). The role of (NH4) 2SO4 consisted primarily of precipitation of plasma proteins that helped to purify the obtained extracts and to form a distinct interface that facilitated subsequent collection of the organic layer. The degree of extraction of the compounds was not significantly affected if the amount of added ammonia solution was increased from 30 to 50 μL (statistically significant differences evaluated using the Student paired t-criterion were not found, p > 0.05). Therefore, addition of ammonia (30 μL) was justified.
Validation of analytical method. Figure 3 shows that the chromatogram of the blank sample (n = 6, blood plasma from six different subjects) did not have interfering peaks at the analyte and IS retention times, which indicated that the used HPLC-MS/MS method was selective. Also, chromatograms of blank samples (n = 6) analyzed immediately after samples with a concentration of 200 ng/mL did not show any signals at the retention times of the tested compounds, which indicated there was no carryover effect.
The LLOQ was 0.30 ng/mL with a signal/noise ratio of 36.8 (n = 6). The average within-run accuracy expressed in % of the nominal concentration and precision represented as the coefficient of variation (CV, %) were 100 and 9%, respectively (Table 3). The detection limit of the analyte was 0.08 ng/mL with a signal/noise ratio of 5.3. Figure 3 shows a representative chromatogram of a sample at the LLOQ level.
Calibration curves were plotted as ratios of analyte peak area to IS peak area as functions of the nominal concentration of the calibration sample in the range from 0.30 to 200 ng/mL using linear regression analysis, particularly a weighted least-squares method (weighting factor 1/x2 , where x is the nominal analyte concentration). A typical calibration function from three analytical cycles obeyed the average equation y = (0.0123 ± 0.0029)x + (0.0017 ± 0.0007) with determination coefficient R2 = (0.9937 ± 0.0017). The accuracy of the determined concentrations of the calibration samples fell in the range from 89 to 114% (of the nominal values).
The averages of within-run and between-run accuracy and precision estimated using QCS1 – 3 and LLOQ samples lay in the range from 97 to 105% (of the nominal values) with CV < 10% (Table 3). The results indicated that the used bioanalytical method was accurate.
The accuracy of the determined analyte concentrations after a two-fold dilution of the samples was (99 ± 9%) of the nominal value with CV = 9.1%. The accuracy for validation of the “dilution by four times” parameter was (93 ± 6)% of the nominal concentration with CV = 6.5%. The accuracy for the reference samples using freshly prepared QCS3 was (106 ± 6)% of the nominal value with CV = 5.7%. Hence, samples with OST concentrations exceeding the established range of the calibration curve could be diluted by two or four times and then analyzed with the allowed accuracy and precision.
The matrix effect was evaluated by comparing analyte and IS peak areas in the presence of the biological matrix (extract of blank sample; plasma from six different subjects) and in its absence (MeCN:H2O = 7:3 v/v). A matrix factor normalized for IS was obtained from the ratio of the analyte matrix factor to the IS matrix factor and was 1.05 ± 0.14 (CV = 13.3%) for QCS1 and 0.94 ± 0.11 (CV = 11.7%) for QCS3. It was difficult to make an unambiguous conclusion about the effect of the studied biological matrix on the analyte signal from the results because it increased slightly in the first instance and was slightly suppressed in the second instance (judging from the given averages). Nevertheless, the results met the established requirements for validation of bioanalytical methods [14, 15].
The stability of OST in human blood plasma (with added NaF and K2C2O4) was studied during long-term (31 d, (80°C) and short-term storage (4 h, room temperature) using QCS1 and QCS3. Also, the effects of freeze(thaw cycles (three cycles, 12 h, from (80°C to room temperature) on the analyte stability in plasma were evaluated. In addition, its stability in the treated biological matrix (in the extract) was studied under autosampler conditions (24 h, 15°C). The reference samples were freshly prepared QCS1 and QCS3. Table 4 shows that the average accuracies of the determined analyte concentrations in all experiments evaluating the stability were in the range from 98 to 108% of the nominal concentrations with CV (11%. The accuracies (from the nominal concentrations) of the reference samples were (106 ± 9)% for QCS1 and (107 ± 6)% for QCS3 with CV < 10% (short-term and long-term stability and stability with freeze(thaw); (105 ± 8)% for QCS1 and (106 ± 6)% for QCS3 with CV < 8% (stability in the autosampler). The results indicated that OST was stable under these storage and sample-preparation conditions.
Analysis of actual sample. The developed and validated method for determining OST in human plasma was successfully tested on 1815 (counting repeated analysis) samples obtained from actual subjects during a bioequivalence study. The difference between OST concentrations established during first and repeated analyses was in the range ±20% for 77% of cases, which met the established requirements (≥67%) [14, 15]. About 270 samples, including blank, null, calibration, and QC samples, were analyzed during a single analytical cycle and indicated that the proposed HPLC-MS/MS method had a high throughput.
References
A. Gupta, S. Guttikar, P. S. Shrivastav, and M. Sanyal, J. Pharm. Anal., 3(3), 149 – 160 (2013).
K. J. Hasson, J. Adv. Pharm. Technol. Res., 13(3), 177 – 181 (2022).
A. I. Kalashnikov, E. G. Sonina, D. A. Kulagina, et al., Pharm. Chem. J., 55(6), 540 – 543 (2021).
P. R. Colunga-Pedraza, S. P. Pena-Lozano, E. Sanchez-Rendon, et al., J. Thromb. Thrombolysis, 54(2), 360 – 366 (2022).
M. Heimerl, T. Gausepohl, J. H. Mueller, and M. Ricke-Hoch, Biology, 11(8), Art. No. 1229 (2022).
H.-J. Qin, S. Li, Y.-B. Zhu, et al., Carbohydr. Res., 520, Art. No. 108631 (2022).
P. Koszalka, A. George, V. Dhanasekaran, et al., mBio, 13(4), Art. No. e0105622 (2022).
N. F. El Azab, Microchem. J., 178, Art. No. 107321 (2022).
W. H. Abdel-Ghaffar and E. A. Abdelghffar, Beni-Suef Univ. J. Basic Appl. Sci., 11(1), Art. No. 15 (2022).
J. Qin, J. Lin, X. Zhang, et al., Front. Pharmacol., 13, Art. No. 849545 (2022).
W. Kromdijk, H. Rosing, M. P. H. van den Broek, et al., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 891 – 892, 57 – 63 (2012).
Z.-Y. Hu, C. Laizure, B. Meibohm, et al., J. Pharm. Biomed. Anal., 72, 245 – 250 (2013).
A. Grigoriev, I. Borisova, I. Yaroshenko, and A. Sidorova, Anal. Bioanal. Chem., 408(14), 3891 – 3897 (2016).
State Pharmacopoeia of the RF, XIVth Ed., FGBU Scientific Centre for Expert Evaluation of Medicinal Products, Ministry of Health of Russia, Moscow (2018) (Appendix No. 6. Requirements for validation of bioanalytical test methods and analysis of tested biological samples).
European Medicines Agency: Guideline on Bioanalytical Method Validation, 2011; https: //www.ema.europa.eu/en/bioanalytical-method-validation (Oct. 25, 2022).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 57, No. 1, pp. 52 – 58, January, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lakeev, A.P., Abdrashitova, N.Y., Bryushinina, O.S. et al. Determination of Oseltamivir in Human Plasma by HPLC-MS/MS. Pharm Chem J 57, 116–122 (2023). https://doi.org/10.1007/s11094-023-02858-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02858-5