Abstract
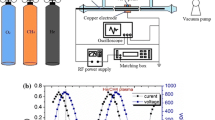
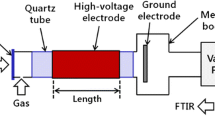
The kinetics of methane decomposition in low frequency (60 Hz) AC arc plasmas was investigated using on-line mass spectrometry and optical emission spectroscopy (OES) in a batch reactor configuration at pressures up to 3 bar absolute. Plasma conversion of CH4 results largely from thermal dissociation and was seen to follow first-order kinetics up to high conversions (> 90%) without observing any rate impedance from reverse hydrocracking. H– and C-atom selectivities for H2, C2H2, and C2H4 were 78% (1.56 mol H2/mol CH4 reacted), 36% (0.18 mol C2H2/mol CH4), and 30% (0.15 mol C2H4/mol CH4), respectively, at 3 bar. In other experiments, H2 diluent concentration played an important role in CH4 dissociation and final product distributions; H abstraction reactions increased the rate of CH4 decomposition at low H2 (yH2 < 0.6) while high H2 (yH2 > 0.6) impeded CH4 decomposition due to hydrocracking of C2 products. The rate of CH4 dissociation was seen to increase with pressure, up to 0.11 mol/m3/s, and the specific energy requirement (SER) decreased with pressure to 365 kJ/mol CH4 at 3 bar. The latter suggests that even higher operating pressures may improve the efficiency of plasma conversion of CH4, and ultimately that plasma pyrolysis may be a viable and energy efficient route to clean (turquoise) H2 and further implementation of chemical process electrification.











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and supporting information documents.
References
Parkinson B, Balcombe P, Speirs JF, Hawkes AD, Hellgardt K (2019) Levelized cost of CO2 mitigation from hydrogen production routes. Energy Environ Sci 12(1):19–40. https://doi.org/10.1039/C8EE02079E
Patlolla SR, Katsu K, Sharafian A, Wei K, Herrera OE, Mérida W (2023) A review of methane pyrolysis technologies for hydrogen production. Renew Sustain Energy Rev 181:113323. https://doi.org/10.1016/j.rser.2023.113323
Ruscic B (2015) Active thermochemical tables: sequential bond dissociation enthalpies of methane, ethane, and methanol and the related thermochemistry. J Phys Chem A 119(28):7810–7837. https://doi.org/10.1021/acs.jpca.5b01346
Olsvik O, Rokstad OA, Holmen A (1995) Pyrolysis of methane in the presence of hydrogen. Chem Eng Technol 18(5):349–358. https://doi.org/10.1002/ceat.270180510
Holliday GC, Exell HC (1929) CXLI—the thermal decomposition of methane. Part I. Decomposition in silica bulbs. J Chem Soc (Resumed). https://doi.org/10.1039/JR9290001066
Li X-S, Zhu A-M, Wang K-J, Xu Y, Song Z-M (2004) Methane conversion to C2 hydrocarbons and hydrogen in atmospheric non-thermal plasmas generated by different electric discharge techniques. Catal Today 98(4):617–624. https://doi.org/10.1016/j.cattod.2004.09.048
Kado S, Sekine Y, Nozaki T, Okazaki K (2004) Diagnosis of atmospheric pressure low temperature plasma and application to high efficient methane conversion. Catal Today 89(1–2):47–55. https://doi.org/10.1016/j.cattod.2003.11.036
Xu C, Tu X (2013) Plasma-assisted methane conversion in an atmospheric pressure dielectric barrier discharge reactor. J Energy Chem 22(3):420–425. https://doi.org/10.1016/S2095-4956(13)60055-8
Scapinello M, Delikonstantis E, Stefanidis GD (2018) Direct methane-to-ethylene conversion in a nanosecond pulsed discharge. Fuel 222:705–710. https://doi.org/10.1016/j.fuel.2018.03.017
Scapinello M, Delikonstantis E, Stefanidis GD (2019) A study on the reaction mechanism of non-oxidative methane coupling in a nanosecond pulsed discharge reactor using isotope analysis. Chem Eng J 360:64–74. https://doi.org/10.1016/j.cej.2018.11.161
Nozaki T, Muto N, Kado S, Okazaki K (2004) Dissociation of vibrationally excited methane on Ni catalyst. Catal Today 89(1–2):57–65. https://doi.org/10.1016/j.cattod.2003.11.040
Lotfalipour R, Ghorbanzadeh AM, Mahdian A (2014) Methane conversion by repetitive nanosecond pulsed plasma. J Phys D: Appl Phys 47(36):365201. https://doi.org/10.1088/0022-3727/47/36/365201
Kado S, Urasaki K, Sekine Y, Fujimoto K, Nozaki T, Okazaki K (2003) Reaction mechanism of methane activation using non-equilibrium pulsed discharge at room temperature. Fuel 82(18):2291–2297. https://doi.org/10.1016/S0016-2361(03)00163-7
Kreuznacht S, Purcel M, Böddeker S, Awakowicz P, **a W, Muhler M, Böke M, von Keudell A (2023) Comparison of the performance of a microwave plasma torch and a gliding arc plasma for hydrogen production via methane pyrolysis. Plasma Processes Polym 20(1):2200132. https://doi.org/10.1002/ppap.202200132
Indarto A, Choi J, Lee H, Song H (2006) Effect of additive gases on methane conversion using gliding arc discharge. Energy 31(14):2986–2995. https://doi.org/10.1016/j.energy.2005.10.034
Fincke JR, Anderson RP, Hyde T, Detering BA, Wright R, Bewley RL, Haggard DC, Swank WD (2002) Plasma thermal conversion of methane to acetylene. Plasma Chem Plasma Process 22(1):105–136. https://doi.org/10.1023/A:1012944615974
Gautier M, Rohani V, Fulcheri L (2017) Direct decarbonization of methane by thermal plasma for the production of hydrogen and high value-added carbon black. Int J Hydrogen Energy 42(47):28140–28156. https://doi.org/10.1016/j.ijhydene.2017.09.021
Polak LS (1967) Low-temperature plasma in petroleum chemistry. Petrol Chem USSR 7(2):136–152. https://doi.org/10.1016/0031-6458(67)90032-9
Bilera IV, Lebedev YuA (2022) Plasma-chemical production of acetylene from hydrocarbons: history and current status (a review). Pet Chem 62(4):329–351. https://doi.org/10.1134/S0965544122010145
Fulcheri L, Rohani V-J, Wyse E, Hardman N, Dames E (2023) An energy-efficient plasma methane pyrolysis process for high yields of carbon black and hydrogen. Int J Hydrogen Energy 48(8):2920–2928. https://doi.org/10.1016/j.ijhydene.2022.10.144
Ohmori Y, Kitamori K, Shimozuma M, Tagashira H (1986) Boltzmann equation analysis of electron swarm behaviour in methane. J Phys D: Appl Phys 19(3):437–455. https://doi.org/10.1088/0022-3727/19/3/013
Kevorkian V, Heath CE, Boudart M (1960) The decomposition of methane in shock waves1. J Phys Chem 64(8):964–968. https://doi.org/10.1021/j100837a002
Kassel LS (1932) The thermal decomposition of methane1. J Am Chem Soc 54(10):3949–3961. https://doi.org/10.1021/ja01349a019
Palmer C, Gordon MJ, Metiu H, McFarland EW (2022) Influence of hydrocarbon feed additives on the high-temperature pyrolysis of methane in molten salt bubble column reactors. React Chem Eng 7(5):1199–1209. https://doi.org/10.1039/D1RE00517K
Heintze M, Magureanu M, Kettlitz M (2002) Mechanism of C2 hydrocarbon formation from methane in a pulsed microwave plasma. J Appl Phys 92(12):7022–7031. https://doi.org/10.1063/1.1521518
Kado S, Sekine Y, Fujimoto K (1999) Direct synthesis of acetylene from methane by direct current pulse discharge. Chem Commun 24:2485–2486. https://doi.org/10.1039/a906914c
Slovetskii DI, Mankelevich YA, Slovetskii SD, Rakhimova TV (2002) Mathematical modeling of the plasma-chemical pyrolysis of methane. High Energy Chem 36:44–52
Ferrari AC, Basko DM (2013) Raman spectroscopy as a versatile tool for studying the properties of graphene. Nature Nanotech 8(4):235–246. https://doi.org/10.1038/nnano.2013.46
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B 61(20):14095–14107. https://doi.org/10.1103/PhysRevB.61.14095
Funding
This material was based upon work supported by the Robert G. Rinker Endowment for Chemical Engineering at UCSB, with auxiliary funding provided by C-Zero, Inc and the National Science Foundation Graduate Research Fellowship under Grant No. 2139319. The results presented made use of the MRL Shared Experimental Facilities of UCSB supported by the MRSEC program (NSF DMR 1720256), a member of the Materials Research Facilities Network (www.mrfn.org), as well as the UCSB Nanofabrication Facility, an open access laboratory.
Author information
Authors and Affiliations
Contributions
NL and MJG conceived and planned experiments. NL carried out experiments, analyzed data, and wrote the draft manuscript. YW assisted with experiments and supplemental information. MJG managed the project and edited the manuscript. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim, N., Wu, Y. & Gordon, M.J. Impact of Pressure and Hydrogen Dilution on the Kinetics of Methane Decomposition in AC-Excited, High Pressure Plasmas. Plasma Chem Plasma Process 44, 47–64 (2024). https://doi.org/10.1007/s11090-023-10416-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10416-w