Abstract
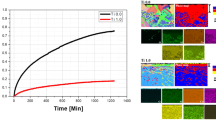
TiAl-based alloys containing relatively high Mn (e.g., Ti–42Al–5Mn (in at.%)) possess excellent hot workability and cost-effective advantages, but may also have poor oxidation resistance at high temperature. Hence, additional attention needs to be paid on the oxidation resistance of these alloys. In this study, a Mn-containing γ-TiAl-based alloy with the composition of Ti–42Al–5Mn has been chosen, and the oxidation behavior of this alloy with different W additions under thermal cycling conditions at 800 °C was investigated. The morphology and the composition distribution of the oxide scale were analyzed by means of scanning electron microscopy equipped with energy-dispersive spectroscopy, X-ray diffractometry and X-ray photoelectron spectroscopy. Auger electron spectroscopy depth profiling was used to investigate the effect of W on the initial stage of oxidation. It shows that the addition of 1 (at.%) W can significantly reduce the oxidation mass gain, and the oxidation reaction rate constant is decreased by an order of magnitude. The adherence of oxide scale is greatly enhanced with no spallation observed in Ti–42Al–5Mn–(0.8, 1)W after 100-h cyclic oxidation. Tungsten promotes the selective oxidation of Al and formation of a denser oxide scale which prevents the inward diffusion of oxygen; in this way, high-temperature oxidation resistance of the alloy is improved.











Similar content being viewed by others
References
H. Clemens and W. Smarsly, Advanced Materials Research278, 551–556 (2011).
M. Yamaguchi, H. Inui and K. Ito, Acta Materialia48, 307–322 (2000).
Y. N. Berdovsky, Intermetallics Research Progress, (Nova Science Publishers, New York, 2008).
Y.-W. Kim and S.-L. Kim, JOM70, 553–560 (2018).
S. Mayer, P. Erdely, F. D. Fischer, et al., Advanced Engineering Materials19, 1600735 (2017).
H. Clemens, W. Wallgram, S. Kremmer, et al., Advanced Engineering Materials10, 707–713 (2008).
T. Tetsui, K. Shindo, S. Kobayashi and M. Takeyama, Scripta Materialia47, 399–403 (2002).
T. Tetsui, K. Shindo, S. Kaji, S. Kobayashi and M. Takeyama, Intermetallics13, 971–978 (2005).
H. Xu, X. B. Li, W. W. **ng, et al., Intermetallics99, 51–58 (2018).
X. B. Li, H. Xu, W. W. **ng, et al., Metals8, 731 (2018).
H. Xu, X. B. Li, W. W. **ng, et al., Advanced Engineering Materials20, 1701059 (2018).
Y. Shida and H. Anada, Oxidation of Metals45, 197–219 (1996).
S. A. Kekare and P. B. Aswath, Journal of Materials Science32, 2485–2499 (1997).
R. Pflumm, S. Friedle and M. Schütze, Intermetallics56, 1–14 (2015).
M. Froehlich, A. Ebach-Stahl, R. Braun and C. Leyens, Materialwissenschaft und Werkstofftechnik38, 667–673 (2007).
K. Zhang, T. B. Zhang, X. H. Zhang and L. Song, Corrosion Science156, 139–146 (2019).
M. Naveed, A. F. Renteria and S. Weiß, Journal of Alloys and Compounds691, 489–497 (2017).
X. Gong, R. R. Chen, H. Z. Fang, et al., Corrosion Science131, 376–385 (2018).
F.-P. **, Q.-M. Hu, A. V. Bakulin, S. E. Kulkova and R. Yang, Intermetallics68, 57–62 (2016).
T. Izumi, T. Yoshioka, S. Hayashi and T. Narita, Intermetallics9, 547–558 (2001).
J. P. Lin, L. L. Zhao, G. Y. Li, et al., Intermetallics19, 131–136 (2011).
R. Pflumm, A. Donchev, S. Mayer, H. Clemens and M. Schütze, Intermetallics53, 45–55 (2014).
Y. Shida and H. Anada, Corrosion Science35, 945–953 (1993).
J. W. Fergus, Materials Science and Engineering: A338, 108–125 (2002).
C. Lang and M. Schutze, Oxidation of Metals46, 255–285 (1996).
T. K. Roy, R. Balasubramaniam and A. Ghosh, Metallurgical and Materials Transactions A27, 3993–4002 (1996).
D. Pilone, F. Felli and A. Brotzu, Intermetallics43, 131–137 (2013).
J. Malecka, Oxidation of Metals91, 365–380 (2019).
M. Hadi, O. Bayat, M. Meratian, A. Shafyei and I. Ebrahimzadeh, Oxidation of Metals90, 421–434 (2018).
Y. Garip and O. Ozdemir, Journal of Alloys and Compounds818, 152818 (2020).
V. A. C. Haanappel, J. D. Sunderkotter and M. F. Stroosnijder, Intermetallics7, 529–541 (1999).
L. L. Zhao, J. P. Lin, Y. L. Wang, F. Ye and G. L. Chen, Acta Metall Sin44, 557–564 (2008).
J. M. **ang, G. B. Mi, S. J. Qu, et al., Scientific Reports8, 12761 (2018).
T. Izumi, T. Yoshioka, S. Hayashi and T. Narita, Intermetallics13, 694–703 (2005).
S. Yuke, S. W. Kim, J. Hahn and D. B. Lee, Oxidation of Metals91, 677–689 (2019).
D. Kim, D. Seo, S. W. Kim, et al., Oxidation of Metals86, 417–430 (2016).
Acknowledgements
The authors thank the National Natural Science Foundation of China for their financial support under contract No. 51971215, the National Natural Science Foundation of Liaoning province of China for their financial support under contract No. 2019-MS-330, and the China postdoctoral science foundation (2019M661152). The authors also thank Prof. Shunnan Zhang in IMR for valuable discussions and Li** Yang in Tsinghua University for her careful analysis and testing.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, P., Li, X., Tang, H. et al. Improved High-Temperature Oxidation Properties for Mn-Containing Beta-Gamma TiAl with W Addition. Oxid Met 93, 433–448 (2020). https://doi.org/10.1007/s11085-020-09964-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-020-09964-9