Abstract
Primordial metabolism co-evolved with the earliest membrane peptides to produce more environmentally fit progeny. Here, we map a continuous, evolutionary path that connects nascent biochemistry with simple, membrane-bound oligopeptides, ion channels and, further, membrane proteins capable of energy transduction and utilization of energy for active transport.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
All terrestrial life is cellular. Membranous boundaries that separate the interior of cells from the environment serve multiple purposes, including protection of synthetic products of the metabolism encapsulated inside cells from dilution in the surrounding medium, forming a two-phase system capable of sustaining chemical and electrical gradients, and facilitating Darwinian evolution (Pohorille and Wilson 1995; Szostak et al. 2001). In most modern theories of the origin of life, it is postulated that protocells - self-assembled, membrane-bound structures that encapsulated the nascent metabolism and information molecules - emerged early in the transition from inanimate to animate matter (for review see Pohorille and Deamer 2009). Initially, the content of protocells was, most likely, highly heterogeneous. This set the stage for their Darwinian evolution. A population of protocells containing material that was capable of producing more environmentally fit progeny would increase in time at the expense of other protocells. In this scenario, protocellular boundaries were inextricably connected to the metabolism they encapsulated: to be inheritable, early metabolism must have led to an increased rate of growth and division of vesicles and, similarly, transport through vesicle boundaries must have supported the evolution of metabolism. Permeation through vesicle walls would have been a robust mechanism to do so, although other mechanisms, such as disruption and subsequent reformation of vesicles were also possible (Deamer and Barchfeld 1982). Everything that could not have been delivered from the environment had to be produced and retained inside protocells. Further, the rates at which the building blocks of different polymers permeated primitive membranes speak to autotrophic vs. heterotrophic origins of metabolism and the identity of the first biopolymers (Sacerdote and Szostak 2005; Wei and Pohorille 2013a). For these reasons, explaining how the coupling between metabolism and membrane-related processes emerged and evolved without the complex machinery of modern cells is one of the key issues in studies of the origin of life.
Consistent with these considerations, our goal is to map a continuous, evolutionary path connected with the nascent biochemistry that led from simple, membrane-bound oligopeptides to ion channels and, further, to membrane proteins capable of energy transduction and utilization of energy for active transport (against concentration gradients.) The last two classes of proteins are at the heart of biology, as they are responsible for kee** cells far from equilibrium, which is an essential property of life.
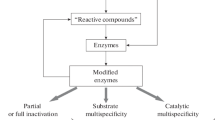
On the basis of recent experimental results and evidence from extant biology, we can conceptually divide this path into five steps of increasing complexity:
-
1.
Membrane-bound dipeptides or short oligopeptides, synthesized inside protocells, promoted faster growth and division of vesicles made of simple amphiphiles,
-
2.
Short, transmembrane peptides induced thinning defects in membranes that increased permeation of substrates for proto-metabolism,
-
3.
The earliest, highly flexible ion channels protected protocells from osmotic disequilibria,
-
4.
Rigid channels relieved osmotic pressure more reliably and led to
-
5.
The emergence of modern channels, receptors, pumps and transporters.
These five steps are illustrated in Fig. 1. In the reminder of this paper, we will briefly describe each of them and present experimental evidence in their support.
Since no robust mechanism for synthesizing phospholipids, which form the walls of modern cells, appears to have been available on the early earth, protocells must have been initially bounded by simpler, amphiphilic material. Protocells made of simple amphiphiles were not just a fleeting phase in evolution. At a minimum, they must have encapsulated metabolism sufficient for synthesis of phospholipids.
-
Step 1. We expect that the coupling between proteins or oligopeptides, membrane-forming molecules, metabolism and information polymers arose at this stage of protocellular evolution, before modern biochemistry was in place. Recently, Adamala and Szostak have demonstrated that such coupling can be achieved with the aid of only very simple peptides (Adamala and Szostak 2013). They encapsulated a Ser-His dipeptide and two terminally blocked amino acids, AcPheOEt and LeuNH2, in oleic-acid vesicles. Ser-His, which is a weak catalyst of peptide bond formation, produced a dipeptide, AcPheLeuNH2, from the amino acid substrates. Vesicles enhanced synthesis of this dipeptide, which bound to the membrane, driving vesicle growth when fatty acids were present in the medium. Vesicles containing AcPheLeuNH2 grew at the expense of those lacking the dipeptide if both were incubated together. As AcPheLeuNH2–containing vesicles became larger they adapted filamentous shapes that directly precede vesicle division. These results demonstrate that encapsulation of a primitive catalyst can drive competition between populations of protocells. Since a non-heritable catalyst was used and the only available substrates were those initially delivered to vesicles, the system was neither self-replicating nor evolving. It would be so, however, if the catalyst were a ribozyme capable of enzymatic or non-enzymatic replication and substrates could permeate the membrane at sufficient rate. Alternative mechanisms that did not involve ribozymes, but rather relied on reproduction in a population (Pohorille 2008) might have been also in play. This demonstrates the feasibility of the first step in the proposed evolutionary path, although it does not necessarily mean that the same amino acids as those used by Adamala and Szostak were protobiologically involved.
-
Step 2. The appearance of phospholipids imparted selective advantage to protocells bounded by phospholipid-containing membranes (Budin and Szostak 2011), eventually driving vesicles made of simple amphiphiles to extinction. Phospholipid membranes, however, are poorly permeable to large solutes and are nearly impermeable to charged species. Yet, the ability to transport ions across membranes was vital for regulating cellular volume, pH homeostasis, generating energy and sensing the environment. Thus the emergence of phospholipid membranes must have been evolutionarily coupled to a mechanism that would keep the encapsulated metabolism well supplied and protocells resistant to environmental stresses. A number of model peptides are known to do so. Many of them bind strongly to the membrane surface and disrupt the membrane, thus causing indiscriminate leakage. RNA molecules that increase membrane permeability act similarly (Vlassov 2005). Another, less disruptive mechanism is mediated by peptides in a transmembrane orientation. If a protein is shorter than the average width of the membrane, the bilayer will locally deform forming a thinning defect in the bilayer, whereby polar lipid head groups and water penetrate the nonpolar membrane interior. The antimicrobial peptide trichogin GAIV, which is built of 10 simple amino acids folded into a helix and spans only half the membrane, induces thinning defects increasing membrane permeability that is both ion-selective and dependent on applied voltage (Bobone et al. 2013). Very similar defects increase permeability of membranes to ions by many orders of magnitude (Wilson and Pohorille 1996).
-
Step 3. To shed light on properties of primordial ion channels that might have evolved from short transmembrane peptides we consider a hexameric channel formed by antiamoebin (AAM), a member of the peptaibol family. AAM is one of the shortest channel-forming peptides, consisting of only 16 amino acids (Duclohier et al. 1998). Its sequence of amino acids contains several α-aminoisobutyric acid and isovaline residues. Interestingly, the same amino acids have been found in meteorites and are assumed to have been common on the early earth. The peptide also acts as an ion carrier, which points to a possible ancestral relation between channel- and carrier-assisted ion transport (Duclohier et al. 1998). Since AAM is not genomically coded it has not been subjected to common evolutionary optimization whereby single-point mutations that improve fitness are retained in the genome of the progeny.
On the basis of molecular dynamics simulations, we found that the AAM channel undergoes unusually large structural fluctuations (Wilson et al. 2011). This suggests that the earliest ion channels were loose bundles of α-helices. The specific identity of amino acids in the helix was less important, a desirable trait in early evolution. The presence of simple, non-standard amino acids that were common in the primordial environment does not prevent the channel from being functional. Also, there is no requirement for amino acids that are synthesized in complex biosynthetic pathways and are assumed to emerge late in protobiological evolution. Taken together, this means that simple ion channels might have existed even before the precise protein synthesis mechanisms or the full suite of amino acids were present, thus protecting nascent cells from osmotic disequilibria.
-
Step 4. From our previous studies it follows that the earliest channels might have been highly flexible, unspecific assemblies of transmembrane peptides. Becoming rigid provided an immediate competitive advantage, as rigid channels better protected protocells from uncontrolled swelling or shrinking, which is often detrimental to their integrity. Two mechanisms can explain how channels acquired rigidity. Neither requires extensive optimization of the amino acid sequence. One possibility is that rigidity was derived from linking individual, channel-forming peptides via short loops. By analogy to subsequent evolution of membrane proteins, this might have happened through gene duplication and fusion in the primordial genome, but other scenarios also exist. In this mechanism, no sequence specificity is required to tame flexibility. Another possibility is that a small number of specific mutations were sufficient to render the channel rigid. It has been recently proposed that a “serine-zipper” motif SXXLXXX, where S stands for serine, L for leucine and X for an arbitrary hydrophobic amino acid, can play this role (North et al. 2006), since only two residue types in a few specific positions are needed to ensure channel stability. A striking example of this architecture is a synthetic channel made entirely of L and S, which is both very stable and ion selective (Lear et al. 1988).
-
Step 5. Acquiring rigidity was the first step on a long evolutionary road to modern membrane proteins that link cellular processes to the environment. The key inventions in this process were the energy transduction system and protein transporters and pumps capable of transporting material against concentration gradients. These two evolutionary advancements, more than anything else, helped to turn simple protocells into exquisite molecular machines that efficiently use external resources for maintenance and growth.
Phylogenetic studies indicate that pumps and transporters evolved from proteins capable of only passive transport. It is unknown, at which stage it took place - could it have happened early on? One way to approach this problem is to create a blueprint of a generic pump and then search for simple channels that might have a similar architecture and, therefore, can be readily turned into a pump. Such a blueprint, based on known biological systems, such as the proton pump bacteriorhodopsin, has been developed for proton pumps (Lanyi and Pohorille 2001). Proton transport is initiated from a proton source located near the center of the bilayer. In response to energy input, for example light, it transfers a proton to the primary proton acceptor, A1. From there the proton is transferred to the secondary acceptor, A2, and possibly further, until it is released to water near the surface of the membrane. The essential requirement for this process is to prevent back transfer of the proton to the proton source, which can be achieved if the transfer from primary to secondary acceptors is fast and practically irreversible. Remarkably, a simple, homotetrameric proton channel M2 from influenza A virus, and its truncated version that consists of only 24 amino acids have a similar architecture (Pinto and Lamb, 2006). In this channel, A1 and A2 are, respectively, histidine and aspartate side chains, and back transfer is prevented by conformational changes in the small region of the channel that comprises the histidines and the neighboring tryptophan residues (Wei and Pohorille 2013b). The only missing element is the proton source. This simple example suggests that the emergence of proton pumps relatively early in evolution was possible.
References
Adamala K, Szostak JW (2013) Competition between model protocells driven by an encapsulated catalyst. Nat Chem 5:495–501
Bobone S, Gerelli Y, De Zotti M, Bocchinfuso G, Farrotti A, Orioni B, Sebastiani F, Latter E, Penfold J, Senesi R, Formaggio F, Palleschi A, Toniolo C, Fragneto G, Stella L (2013) Membrane thickness and the mechanism of action of the short peptaibol trichogin GA IV. Biochim Biophys Acta 1828:1013–1024
Budin I, Szostak JW (2011) Physical effects underlying the transition from primitive to modern cell membranes. Proc Natl Acad Sci U S A 108:5249–5254
Deamer DW, Barchfeld GL (1982) Encapsulation of macromolecules by lipid vesicles under simulated prebiotic conditions. J Mol Evol 18:203–206
Duclohier H, Snook CF, Wallace BA (1998) Antiamoebin can function as a carrier or as a pore-forming peptaibol. Biochim Biophys Acta 1415:255–260
Lanyi JK, Pohorille A (2001) Proton pumps: mechanism of action and applications. Trends Biotechnol 19:140–144
Lear JD, Wasserman ZR, DeGrado WF (1988) Synthetic amphiphilic peptide models for protein ion channels. Science 240:1177–1181
North B, Cristian L, Fu Stowell X, Lear JD, Saven JG, Degrado WF (2006) Characterization of a membrane protein folding motif, the Ser zipper, using designed peptides. J Mol Biol 359:930–939
Pinto LH, Lamb RA (2006) The M2 proton channels of influenza A and B viruses. J Biol Chem 281:8997-9000
Pohorille A (2008) Protocells as universal ancestors of living systems. In: Rasmussen S, Bedau M, Chen L, Deamer D, Krakauer D, Packard N, Stadler P (eds) Protocells: bridging nonliving and living matter. MIT Press, Cambridge, pp 563–582
Pohorille A, Deamer D (2009) Self-assembly and function of primitive cell membranes. Res Microbiol 160:449–456
Pohorille A, Wilson MA (1995) Molecular dynamics studies of simple membrane water interfaces — structure and functions in the beginnings of cellular life. Orig Life Evol Biosph 25:21–46
Sacerdote MG, Szostak JW (2005) Semipermeable lipid bilayers exhibit diastereoselectivity favoring ribose. Proc Natl Acad Sci U S A 102:6004–6008
Szostak JW, Luisi PL, Bartel DP (2001) Synthesizing life. Nature 409:387–390
Vlassov A (2005) How was membrane permeability produced in an RNA world? Orig Life Evol Biosph 35:135–149
Wei C, Pohorille A (2013a) Permeation of aldopentoses and nucleosides through fatty acid and phospholipid membranes: implications to the origins of life. Astrobiology 13:177–188
Wei C, Pohorille A (2013b) Activation and proton transport mechanism in influenza A M2 channel. Biophys J 105:2036–2045
Wilson MA, Pohorille A (1996) Mechanism of unassisted ion transport across membrane bilayers. J Am Chem Soc 118:6580–6587
Wilson MA, Wei C, Bjelkmar P, Wallace BA, Pohorille A (2011) Molecular dynamics simulation of the antiamoebin ion channel: linking structure and conductance. Biophys J 100:2394–402
Acknowledgments
This work was supported by the NASA Exobiology Program and the NASA Astrobiology Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
A. Wilson, M., Wei, C. & Pohorille, A. Towards Co-Evolution of Membrane Proteins and Metabolism. Orig Life Evol Biosph 44, 357–361 (2014). https://doi.org/10.1007/s11084-014-9393-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-014-9393-2