Abstract
The development of methods for in vivo detection of cerebral beta amyloid retention and tau accumulation have been increasingly useful in characterizing preclinical Alzheimer’s disease (AD). While the association between these biomarkers and eventual AD has been demonstrated among cognitively intact older adults, the link between biomarkers and neurocognitive ability remains unclear. We conducted a meta-analysis to test the hypothesis that cognitively intact older adults would show statistically discernable differences in neuropsychological performance by amyloid status (amyloid negative = A-, amyloid positive = A+). We secondarily hypothesized a third group characterized by either CSF tau pathology or neurodegeneration, in addition to amyloidosis (A+/N+ or Stage 2), would show lower neuropsychology scores than the amyloid positive group (A+/N- or Stage 1) when compared to the amyloid negative group. Pubmed, PsychINFO, and other sources were searched for relevant articles, yielding 775 total sources. After review for inclusion/exclusion criteria, duplicates, and risk of bias, 61 studies were utilized in the final meta-analysis. Results showed A+ was associated with poorer performance in the domains of global cognitive function, memory, language, visuospatial ability, processing speed, and attention/working memory/executive functions when compared to A-. A+/N+ showed lower performances on memory measures when compared to A+/N- in secondary analyses based on a smaller subset of studies. Results support the notion that neuropsychological measures are sensitive to different stages of preclinical AD among cognitively intact older adults. Further research is needed to determine what constitutes meaningful differences in neuropsychological performance among cognitively intact older adults.

















Similar content being viewed by others
Change history
23 December 2017
Errors were discovered in the reporting of processing speed data that do not impact the interpretation of findings.
References
Aizenstein, H. J., Nebes, R. D., Saxton, J. A., et al. (2008). Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of Neurology, 65(11), 1509–1517.
Alcolea, D., Martínez-Lage, P., Sánchez-Juan, P., et al. (2015). Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology, 85(7), 626–633.
Amariglio, R. E., Becker, J. A., Carmasin, J., et al. (2012). Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia, 50, 2880–2886.
Amariglio, R. E., Mormino, E. C., Pietras, A. C., et al. (2015). Subjective cognitive concerns, amyloid-β, and neurodegeneration in clinically normal elderly. Neurology, 85(1), 56–62.
Andreasen, N., Minthon, L., Davidsson, P., et al. (2001). Evaluation of CSF-tau and CSF-Aβ42 as diagnostic markers for Alzheimer disease in clinical practice. Archives of Neurology, 58, 373–379.
Ayutyanont, N., Langbaum, J. B., Hendrix, S. B., et al. (2014). The Alzheimer’s Precention initiative composite cognitive test score: Sample size estimates for the evaluation of preclinical Alzheimer’s disease treatments in presenilin 1 E280A mutation carriers. The Journal of Clinical Psychiatry, 75, 652–660.
Besson, F. L., La Joie, R., Doeuvre, L., et al. (2015). Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer's disease. The Journal of Neuroscience, 35(29), 10402–10411.
Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2009). Introduction to meta-analysis. West Sussex: Wiley.
Braak, H., & Del Tredici, K. (2015). The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain, 138, 2814–2833.
Buckley, R. F., Maruff, P., Ames, D., et al. (2016). Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer's disease. Alzheimer’s & Dementia, 12(7), 796–804.
Chen, K., Roontiva, A., Thiyyagura, P., et al. (2015). Improved power for characterizing longitudinal amyloid-β PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. The Journal of Nuclear Medicine, 56(4), 560–566.
Chételat, G., Villemagne, V. L., Pike, K. E., et al. (2010). Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain, 133(11), 3349–3358.
Chételat, G., Villemagne, V. L., Villain, N., et al. (2012). Accelerated cortical atrophy in cognitively normal elderly with high β-amyloid deposition. Neurology, 78(7), 477–484.
Cochran, W. G. (1954). The combination of estimates from different experiments. Biometrics, 1, 101–129.
Donohue, M. C., Sperling, R. A., Salmon, D. P., et al. (2014). The preclinical Alzheimer cognitive composite: Measuring amyloid-related decline. JAMA Neurology, 71(8), 961–970.
Doraiswamy, P. M., Sperling, R. A., Coleman, R. E., et al. (2012). Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline: A multicenter study. Neurology, 79(16), 1636–1644.
Doraiswamy, P. M., Sperling, R. A., Johnson, K., et al. (2014). Florbetapir F 18 amyloid PET and 36-month cognitive decline: A prospective multicenter study. Molecular Psychiatry, 19(9), 1044–1051.
Dubois, B., Hampel, H., Feldman, H. H., Scheltens, P., Aisen, P., Andrieu, S., Jack, C. R. (2016). Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s and Dementia, 12, 292–323.
Duff, K., Norman, N. L., & Hoffman, J. M. (2014). Practice effects and amyloid deposition: Preliminary data on a method for enriching samples in clinical trials. Alzheimer’s Dis Assoc Disord, 28, 247–252.
Edmonds, E. C., Delano-Wood, L., Galasko, D. R., et al. (2015). Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. Journal of Alzheimer’s Disease, 47, 231–242.
Elman, J. A., Oh, H., Madison, C. M., et al. (2014). Neural compensation in older people with brain amyloid-β deposition. Nature Neuroscience, 17(10), 1316–1318.
Fortea, J., Sala-Llonch, R., Bartrés-Faz, D., et al. (2011). Cognitively preserved subjects with transitional cerebrospinal fluid ß-amyloid 1-42 values have thicker cortex in Alzheimer's disease vulnerable areas. Biological Psychiatry, 70(2), 183–190.
Fripp, J., Bourgeat, P., Acosta, O., et al. (2008). Appearance modeling of 11C PiB PET images: Characterizing amyloid deposition in Alzheimer's disease, mild cognitive impairment and healthy aging. NeuroImage, 43(3), 430–439.
Gidicsin, C. M., Maye, J. E., Locascio, J. J., et al. (2015). Cognitive activity relates to cognitive performance but not to Alzheimer disease biomarkers. Neurology, 85(1), 48–55.
Gietl, A. F., Warnock, G., Riese, F., et al. (2015). Regional cerebral blood flow estimated by early PiB uptake is reduced in mild cognitive impairment and associated with age in an amyloid-dependent manner. Neurobiology of Aging, 36(4), 1619–1628.
Goldman, W. P., Price, J. L., Storandt, M., et al. (2001). Absence of cognitive impairment or decline in preclinical Alzheimer’s disease. Neurology, 56, 361–367.
Gu, Y., Razlighi, Q. R., Zahodne, L. B., et al. (2015). Brain amyloid deposition and longitudinal cognitive decline in Nondemented older subjects: Results from a multi-ethnic population. PloS One, 10(7), e0123743.
Hardy, J. A., & Higgins, G. A. (1992). Alzheimer’s disease: The amyloid cascade hypothesis. Science, 256, 184–185.
Hardy, J., & Selkoe, D. J. (2002). The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science, 297, 353–356.
Harrington, K. D., Gould, E., Lim, Y. Y., et al. (2016). Amyloid burden and incident depressive symptoms in cognitively normal older adults. International Journal of Geriatric Psychiatry. Advance online publication. doi:10.1002/gps.4489.
Hassenstab, J., Monsell, S. E., Mock, C., et al. (2015). Neuropsychological markers of cognitive decline in persons with Alzheimer disease neuropathology. J Neuropath Exp Neurol, 74, 1086–1092.
Hassenstab, J., Chasse, R., Grabow, P., et al. (2016). Certified normal: Alzheimer’s disease biomarkers and normative estimates of cognitive functioning. Neurobiology of Aging, 43, 23–33.
Hatashita, S., & Yamasaki, H. (2010). Clinically different stages of Alzheimer's disease associated by amyloid deposition with [11C]-PIB PET imaging. Journal of Alzheimer’s Disease, 21(3), 995–1003.
Hedden, T., Oh, H., Younger, A. P., & Patel, T. A. (2013). Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology, 80(14), 1341–1348.
Hedges, L. V. (1981). Distribution theory for Glass's estimator of effect size and related estimators. Journal of Educational Statistics, 6(2), 107–128.
Hedges, L. V., & Vevea, J. L. (1998). Fixed- and random-effects models in meta-analysis. Psychological Methods, 4, 486–504.
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analysis. BMJ, 327, 557–560.
Holland, D., McEvoy, L. K., Desikan, R. S., et al. (2012). Enrichment and stratification for Predementia Alzheimer disease clinical trials. PloS One, 7(10), e47739.
Hsu, P. J., Shou, H., Benzinger, T., et al. (2014). Amyloid burden in cognitively normal elderly is associated with preferential hippocampal subfield volume loss. Journal of Alzheimer’s Disease, 45(1), 27–33.
Huijbers, W., Mormino, E. C., Wigman, S. E., et al. (2014). Amyloid deposition is linked to aberrant entorhinal activity among cognitively normal older adults. The Journal of Neuroscience, 34(15), 5200–5210.
Iturria-Medina, Y., Sotero, R. C., & Toussaint, P. J. (2016). Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nature Communications, 7, article number: 11934.
Jack Jr., C. R., Knopman, D. S., Jagust, W. J., et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology, 9(1), 119–128.
Jack Jr., C. R., Knopman, D. S., Weigand, S. D., et al. (2012). An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Annals of Neurology, 71, 765–775.
Jack Jr., C. R., Knopman, D. S., Jagust, W. J., et al. (2013a). Update on hypothetical model of Alzheimer’s disease biomarkers. Lancet Neurology, 12(2), 207–216.
Jack Jr., C. R., Wiste, H. J., Weigand, S. D., et al. (2013b). Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology, 81(20), 1732–1740.
Jack Jr., C. R., Wiste, H. J., Weigand, S. D., et al. (2014). Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: A cross-sectional study. Lancet Neurology, 13(10), 997–1005.
Jansen, W. J., Ossenkoppele, R., Knol, D. L., Tijms, B. M., Scheltens, P., Verhey, F. R. J., Visser, P. J., & Amyloid Biomarker Study Group. (2015). Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA, 313(19), 1924–1938.
Jedynak, B. M., Lang, A., Liu, B., et al. (2012). A computational neurodegenerative diease progression score: Method and results with the Alzheimer’s Disease Neuroimaging Initiative cohort. NeuroImage, 63, 1478–1486.
Jessen, F., Amariglio, R. E., van Boxtel, M., et al. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 10, 844–852.
Knopman, D. S., Jack Jr., C. R., Wiste, H. J., et al. (2012). Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology, 78(20), 1576–1582.
Knopman, D. S., Beiser, A., Machulda, M. M., et al. (2015). Spectrum of cognition short of dementia: Framingham heart study and Mayo Clinic study of aging. Neurology, 85, 1712–1721.
Lamar, M., Resnick, S. M., & Zonderman, A. B. (2003). Longitudinal changes in verbal memory in older adults. Neurology, 60, 82–86.
Langbaum, J. B., Hendrix, S. B., Ayutyanont, N., et al. (2014). An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 10, 666–674.
Langbaum, J. B., Hendrix, S. B., Ayutyanont, N., et al. (2015). Establishing composite cognitive endpoints for use in preclinical Alzheimer’s disease trials. The Journal of Prevention of Alzheimer’s Disease, 2(1), 2–3.
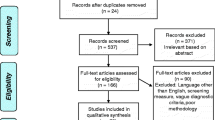
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P. A., Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine, 6, e1000100.
Lim, H. K., Nebes, R., Snitz, B., et al. (2014). Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects. Brain, 137, 3327–3338.
Lim, Y. Y., Maruff, P., Schindler, R., et al. (2015). Disruption of cholinergic neurotransmission exacerbates Aβ-related cognitive impairment in preclinical Alzheimer’s disease. Neurobiology of Aging, 36, 2709–2715.
Lim, Y. Y., Snyder, P. J., Pietrzak, R. H., et al. (2016). Sensitivity of composite scores to amyloid burden in preclinical Alzheimer's disease: Introducing the Z-scores of attention, verbal fluency, and episodic memory for Nondemented older adults composite score. Alzheimer’s & Dementia, 2, 19–26.
Llado-Saz, S., Atienzam, M., & Cantero, J. L. (2015). Increased levels of plasma amyloid-beta are related to cortical thinning and cognitive decline in cognitively normal elderly subjects. Neurobiology of Aging, 36(10), 2791–2797.
Machulda, M. M., Hagen, C. E., Wiste, H. J., et al. (in press). Practice effects and longitudinal cognitive change in clinically normal older adutls differ by Alzheimer imaging biomarker status. The Clinical Neuropsychologist. doi:10.1080/13854046.2016.1241303.
Marchant, N. L., Reed, B. R., Sanossian, N., et al. (2013). The aging brain and cognition: Contribution of vascular injury and aβ to mild cognitive dysfunction. JAMA Neurology, 70(4), 488–495.
Mathis, C. A., Kuller, L. H., Klunk, W. E., et al. (2013). In vivo assessment of amyloid-β deposition in nondemented very elderly subjects. Annals of Neurology, 73, 751–761.
Molinuevo, J. L., Ripolles, P., Simó, M., et al. (2014). White matter changes in preclinical Alzheimer's disease: A magnetic resonance imaging-diffusion tensor imaging study on cognitively normal older people with positive amyloid β protein 42 levels. Neurobiology of Aging, 35(12), 2671–2680.
Mormino, E. C., Brandel, M. G., Madison, C. M., et al. (2012). Not quite PIB-positive, not quite PIB-negative: Slight PIB elevations in elderly normal control subjects are biologically relevant. NeuroImage, 59, 1152–1160.
Nelson, P. T., Alafuzoff, I., Bigio, E. H., Bouras, C., Braak, H., Cairns, N. J., et al. (2012). Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. Journal of Neuropathology & Experimental Neurology, 71, 362–381.
Oh, H., Mormino, E. C., Madison, C., et al. (2010). β-amyloid affects frontal and posterior brain networks in normal aging. NeuroImage, 54, 1887–1895.
Oh, H., Madison, C., Haight, T. J., et al. (2012). Effects of age and β-amyloid on cognitive changes in normal elderly people. Neurobiology of Aging, 33(12), 2746–2755.
Oh, H., Steffener, J., Razlighi, Q. R., et al. (2015). Aβ-related hyperactivation in frontoparietal control regions in cognitively normal elderly. Neurobiology of Aging, 36(12), 3247–3254.
Oh, H., Steffener, J., Razlighi, Q. R., et al. (2016). β-amyloid deposition is associated with decreased right prefrontal activation during task switching among cognitively normal elderly. Journal of Neuroscience, 36(6), 1962–1970.
Ossenkoppele, R., Madison, C., Oh, H., et al. (2014). Is verbal episodic memory in elderly with amyloid deposits preserved through altered neuronal function? Cerebral Cortex, 24(8), 2210–2218.
Petersen, R. C., Wiste, H. J., Weigand, S. D., et al. (2016). Association of Elevated Amyloid Levels with Cognition and Biomarkers in cognitively normal people from the community. JAMA Neurology, 73(1), 85–92.
Pike, K. E., Ellis, K. A., Villemagne, V. L., et al. (2011). Cognition and beta-amyloid in preclinical Alzheimer's disease: Data from the AIBL study. Neuropsychologia, 49(9), 2384–2390.
Rentz, D. M., Locascio, J. J., Becker, J. A., et al. (2010). Cognition, reserve, and amyloid deposition in normal aging. Annals of Neurology, 67, 353–364.
Schott, J. M., Bartlett, J. W., Fox, N. C., & Barnes, J. (2010). Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Aβ1-42. Annals of Neurology, 68(6), 825–834.
Snitz, B. E., Weissfeld, L. A., Lopez, O. L., et al. (2013). Cognitive trajectories associated with β-amyloid deposition in the oldest-old without dementia. Neurology, 80(15), 1378–1384.
Soldan, A., Pettigrew, C., Cai, Q., et al. (2016). Hypothetical preclinical Alzheimer disease groups and longitudinal cognitive change. JAMA Neurology, 73(6), 698–705.
Sperling, R. A., Aisen, P. S., Beckett, L. A., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 280–292.
Sperling, R. A., Johnson, K. A., Doraiswamy, P. M., et al. (2013). Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiology of Aging, 34(3), 822–831.
Stark, S. L., Roe, C. M., Grant, E. A., et al. (2013). Preclinical Alzheimer disease and risk of falls. Neurology, 81, 437–443.
Susanto, T. A., Pua, E. P., & Zhou, J. (2015). Cognition, brain atrophy, and cerebrospinal fluid biomarkers changes from preclinical to dementia stage of Alzheimer's disease and the influence of apolipoprotein e. Journal of Alzheimer’s Disease, 45(1), 253–268.
Thai, C., Lim, Y. Y., Villemagne, V. L., et al. (2015). Amyloid-related memory decline in preclinical Alzheimer's disease is dependent on APOE ε4 and is detectable over 18-months. PloS One, 10(10), e0139082.
Vemuri, P., Lesnick, T. G., Przybelski, S. A., et al. (2015). Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain, 138, 761–771.
Villemagne, V. L., Burnham, S., Bourgeat, P., et al. (2013). Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: A prospective cohort study. Lancet Neurology, 12(4), 357–367.
Villeneuve, S., Reed, B. R., Wirth, M., et al. (2014). Cortical thickness mediates the effect of β-amyloid on episodic memory. Neurology, 82(9), 761–767.
Viola, K. L., & Klein, W. L. (2015). Amyloid B oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathologica, 129, 183–206.
Vlassenko, A. G., McCue, L., Jasielec, M. S., et al. (2016). Imaging and cerebrospinal fluid biomarkers in early preclinical alzheimer disease. Annals of Neurology, 80(3), 379–387.
Voevodskaya, O., Sundgren, P. C., Strandberg, O., et al. (2016). Myo-inositol changes precede amyloid pathology and relate to APOE genotype in Alzheimer disease. Neurology, 86(19), 1754–1761.
Vos, S. J., **ong, C., Visser, P. J., et al. (2013). Preclinical Alzheimer's disease and its outcome: A longitudinal cohort study. Lancet Neurology, 12(10), 957–965.
Vos, S. J., Gordon, B. A., Su, Y., et al. (2016). NIA-AA staging of preclinical Alzheimer disease: Discordance and concordance of CSF and imaging biomarkers. Neurobiology of Aging, 44, 1–8.
Wirth, M., Madison, C. M., Rabinovici, G. D., et al. (2013a). Alzheimer’s disease neurodegenerative biomarkers are associated with decreased cognitive function but not β-amyloid in cognitively normal older individuals. Neurobiology of Disease, 33(13), 5553–5563.
Wirth, M., Oh, H., Mormino, E. C., et al. (2013b). The effect of amyloid β on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimer’s & Dementia, 9(6), 687–698.
Acknowledgments
SDH is supported by National Institute on Aging grant K23AG040625, and the American Federation for Aging Research (AFAR). NHS serves as a consultant to Biogen. The funding agencies had no role in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
A correction to this article is available online at https://doi.org/10.1007/s11065-017-9366-0.
Rights and permissions
About this article
Cite this article
Duke Han, S., Nguyen, C.P., Stricker, N.H. et al. Detectable Neuropsychological Differences in Early Preclinical Alzheimer’s Disease: A Meta-Analysis. Neuropsychol Rev 27, 305–325 (2017). https://doi.org/10.1007/s11065-017-9345-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-017-9345-5