Abstract
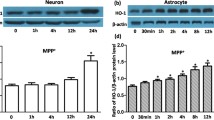
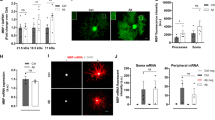
Parkinson’s disease (PD) is a prevalent neurodegenerative disorder characterized by the loss of dopaminergic neurons and the accumulation of iron in the substantia nigra. While iron accumulation and inflammation are implicated in PD pathogenesis, their impact on oligodendrocytes, the brain’s myelin-forming cells, remains elusive. This study investigated the influence of interleukin-1β (IL-1β), an elevated proinflammatory cytokine in PD, on iron-related proteins in MO3.13 oligodendrocytes. We found that IL-1β treatment in undifferentiated MO3.13 oligodendrocytes increased iron regulatory protein 1 and transferrin receptor 1 (TfR1) expression while decreasing ferroportin 1 (FPN1) expression. Consequently, iron uptake was enhanced, and iron release was reduced, leading to intracellular iron accumulation. Conversely, IL-1β treatment in differentiated MO3.13 oligodendrocytes exhibited the opposite effect, with decreased TfR1 expression, increased FPN1 expression, and reduced iron uptake. These findings suggest that IL-1β-induced dysregulation of iron metabolism in oligodendrocytes may contribute to the pathological processes observed in PD. IL-1β can increase the iron content in undifferentiated oligodendrocytes, thus facilitating the differentiation of undifferentiated MO3.13 oligodendrocytes. In differentiated oligodendrocytes, IL-1β may facilitate iron release, providing a potential source of iron for neighboring dopaminergic neurons, thereby initiating a cascade of deleterious events. This study provides valuable insights into the intricate interplay between inflammation, abnormal iron accumulation, and oligodendrocyte dysfunction in PD. Targeting the IL-1β-mediated alterations in iron metabolism may hold therapeutic potential for mitigating neurodegeneration and preserving dopaminergic function in PD.






Similar content being viewed by others
Data Availability
All data analyzed in this study are included within the article.
References
Tolosa E, Garrido A, Scholz SW, Poewe W (2021) Challenges in the diagnosis of Parkinson’s Disease. Lancet Neurol 20:385–397. https://doi.org/10.1016/S1474-4422(21)00030-2
Weintraub D, Aarsland D, Chaudhuri KR et al (2022) The neuropsychiatry of Parkinson’s Disease: advances and challenges. Lancet Neurol 21:89–102. https://doi.org/10.1016/S1474-4422(21)00330-6
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. Lancet 397:2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X
Auguste YSS, Ferro A, Kahng JA et al (2022) Oligodendrocyte precursor cells engulf synapses during circuit remodeling in mice. Nat Neurosci 25:1273–1278. https://doi.org/10.1038/s41593-022-01170-x
Azevedo C, Teku G, Pomeshchik Y et al (2022) Parkinson’s disease and multiple system atrophy patient iPSC-derived oligodendrocytes exhibit alpha-synuclein-induced changes in maturation and immune reactive properties. Proc Natl Acad Sci USA 119:e2111405119. https://doi.org/10.1073/pnas.2111405119
Chamberlain KA, Huang N, **e Y et al (2021) Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron 109:3456-3472e8. https://doi.org/10.1016/j.neuron.2021.08.011
Cheli VT, Correale J, Paez PM, Pasquini JM (2020) Iron metabolism in oligodendrocytes and astrocytes, implications for myelination and remyelination. ASN Neuro 12:1759091420962681. https://doi.org/10.1177/1759091420962681
Annese V, Barcia C, Ros-Bernal F et al (2013) Evidence of oligodendrogliosis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Neuropathol Appl Neurobiol 39:132–143. https://doi.org/10.1111/j.1365-2990.2012.01271.x
Bryois J, Skene NG, Hansen TF et al (2020) Genetic identification of cell types underlying brain complex traits yields insights into the etiology of Parkinson’s disease. Nat Genet 52:482–493. https://doi.org/10.1038/s41588-020-0610-9
Wang GS, Eriksson LC, **a L et al (1999) Dietary iron overload inhibits carbon tetrachloride-induced promotion in chemical hepatocarcinogenesis: effects on cell proliferation, apoptosis, and antioxidation. J Hepatol 30:689–698. https://doi.org/10.1016/s0168-8278(99)80201-3
Masaldan S, Bush AI, Devos D et al (2019) Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic Biol Med 133:221–233. https://doi.org/10.1016/j.freeradbiomed.2018.09.033
Sofic E, Riederer P, Heinsen H et al (1988) Increased iron (III) and total iron content in post mortem substantia nigra of Parkinsonian brain. J Neural Transm 74:199–205. https://doi.org/10.1007/BF01244786
Reimão S, Ferreira S, Nunes RG et al (2016) Magnetic resonance correlation of iron content with neuromelanin in the substantia nigra of early-stage Parkinson’s Disease. Eur J Neurol 23:368–374. https://doi.org/10.1111/ene.12838
Park CH, Valore EV, Waring AJ, Ganz T (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276:7806–7810. https://doi.org/10.1074/jbc.M008922200
Wang S-M, Fu L-J, Duan X-L et al (2010) Role of hepcidin in murine brain iron metabolism. Cell Mol Life Sci 67:123–133. https://doi.org/10.1007/s00018-009-0167-3
Ganz T (2005) Cellular iron: ferroportin is the only way out. Cell Metab 1:155–157. https://doi.org/10.1016/j.cmet.2005.02.005
Nemeth E, Tuttle MS, Powelson J et al (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093. https://doi.org/10.1126/science.1104742
Anderson SA, Nizzi CP, Chang Y-I et al (2013) The IRP1-HIF-2α axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption. Cell Metab 17:282–290. https://doi.org/10.1016/j.cmet.2013.01.007
Meyron-Holtz EG, Ghosh MC, Rouault TA (2004) Mammalian tissue oxygen levels modulate iron-regulatory protein activities in vivo. Science 306:2087–2090. https://doi.org/10.1126/science.1103786
Dev S, Kumari S, Singh N et al (2015) Role of extracellular hydrogen peroxide in regulation of iron homeostasis genes in neuronal cells: implication in iron accumulation. Free Radic Biol Med 86:78–89. https://doi.org/10.1016/j.freeradbiomed.2015.05.025
Hentze MW, Muckenthaler MU, Galy B, Camaschella C (2010) Two to tango: regulation of mammalian iron metabolism. Cell 142:24–38. https://doi.org/10.1016/j.cell.2010.06.028
Cheli VT, Santiago González DA, Wan Q et al (2021) H-ferritin expression in astrocytes is necessary for proper oligodendrocyte development and myelination. Glia 69:2981–2998. https://doi.org/10.1002/glia.24083
Chiou B, Neely EB, Mcdevitt DS et al (2020) Transferrin and H-ferritin involvement in brain iron acquisition during postnatal development: impact of sex and genotype. J Neurochem 152:381–396. https://doi.org/10.1111/jnc.14834
You Y, Muraoka S, Jedrychowski MP et al (2022) Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer’s disease brain. J Extracell Vesicles 11:e12183. https://doi.org/10.1002/jev2.12183
Sun M-F, Zhu Y-L, Zhou Z-L et al (2018) Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun 70:48–60. https://doi.org/10.1016/j.bbi.2018.02.005
Liu T-W, Chen C-M, Chang K-H (2022) Biomarker of neuroinflammation in Parkinson’s disease. Int J Mol Sci 23:4148. https://doi.org/10.3390/ijms23084148
Bottigliengo D, Foco L, Seibler P et al (2022) A mendelian randomization study investigating the causal role of inflammation on Parkinson’s disease. Brain 145:3444–3453. https://doi.org/10.1093/brain/awac193
Sterling JK, Kam T-I, Guttha S et al (2022) Interleukin-6 triggers toxic neuronal iron sequestration in response to pathological α-synuclein. Cell Rep 38:110358. https://doi.org/10.1016/j.celrep.2022.110358
Miao W, Zhao Y, Huang Y et al (2020) IL-13 ameliorates neuroinflammation and promotes functional recovery after traumatic brain injury. J Immunol 204:1486–1498. https://doi.org/10.4049/jimmunol.1900909
McNamara NB, Munro DAD, Bestard-Cuche N et al (2023) Microglia regulate central nervous system myelin growth and integrity. Nature 613:120–129. https://doi.org/10.1038/s41586-022-05534-y
Li Q, Lan X, Han X et al (2021) Microglia-derived interleukin-10 accelerates post-intracerebral hemorrhage hematoma clearance by regulating CD36. Brain Behav Immun 94:437–457. https://doi.org/10.1016/j.bbi.2021.02.001
Bajbouj K, Shafarin J, Muhammad JS et al (2020) Estrogen signaling differentially alters iron metabolism in monocytes in an interleukin 6-dependent manner. Immunobiology 225:151995. https://doi.org/10.1016/j.imbio.2020.151995
Zhou S, Du X, **e J, Wang J (2017) Interleukin-6 regulates iron-related proteins through c-Jun N-terminal kinase activation in BV2 microglial cell lines. PLoS One 12:e0180464. https://doi.org/10.1371/journal.pone.0180464
Wang J, Song N, Jiang H et al (2013) Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim Biophys Acta 1832:618–625. https://doi.org/10.1016/j.bbadis.2013.01.021
Filipovic R, Zecevic N (2005) Lipopolysaccharide affects golli expression and promotes proliferation of oligodendrocyte progenitors. Glia 49:457–466. https://doi.org/10.1002/glia.20125
Kroner A, Greenhalgh AD, Zarruk JG et al (2014) TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83:1098–1116. https://doi.org/10.1016/j.neuron.2014.07.027
Cheng J, Liao Y, Dong Y et al (2020) Microglial autophagy defect causes parkinson disease-like symptoms by accelerating inflammasome activation in mice. Autophagy 16:2193–2205. https://doi.org/10.1080/15548627.2020.1719723
Leal MC, Casabona JC, Puntel M, Pitossi FJ (2013) Interleukin-1β and tumor necrosis factor-α: reliable targets for protective therapies in Parkinson’s disease? Front Cell Neurosci 7:53. https://doi.org/10.3389/fncel.2013.00053
Colonna M, Brioschi S (2020) Neuroinflammation and neurodegeneration in human brain at single-cell resolution. Nat Rev Immunol 20:81–82. https://doi.org/10.1038/s41577-019-0262-0
Peferoen L, Kipp M, van der Valk P et al (2014) Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 141:302–313. https://doi.org/10.1111/imm.12163
Lee NJ, Ha S-K, Sati P et al (2019) Potential role of iron in repair of inflammatory demyelinating lesions. J Clin Invest 129:4365–4376. https://doi.org/10.1172/JCI126809
Camargo N, Goudriaan A, van Deijk A-LF et al (2017) Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol 15:e1002605. https://doi.org/10.1371/journal.pbio.1002605
Tansey MG, McCoy MK, Frank-Cannon TC (2007) Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol 208:1–25. https://doi.org/10.1016/j.expneurol.2007.07.004
Nemeth E, Ganz T (2023) Hepcidin and iron in health and disease. Annu Rev Med 74:261–277. https://doi.org/10.1146/annurev-med-043021-032816
Rouault TA, Tong W-H (2005) Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol 6:345–351. https://doi.org/10.1038/nrm1620
Lepka K, Volbracht K, Bill E et al (2017) Iron-sulfur glutaredoxin 2 protects oligodendrocytes against damage induced by nitric oxide release from activated microglia. Glia 65:1521–1534. https://doi.org/10.1002/glia.23178
Kühn LC (2015) Iron regulatory proteins and their role in controlling iron metabolism. Metallomics 7:232–243. https://doi.org/10.1039/c4mt00164h
Huynh N, Ou Q, Cox P et al (2019) Glycogen branching enzyme controls cellular iron homeostasis via iron regulatory protein 1 and mitoNEET. Nat Commun 10:5463. https://doi.org/10.1038/s41467-019-13237-8
Yao F, Cui X, Zhang Y et al (2021) Iron regulatory protein 1 promotes ferroptosis by sustaining cellular iron homeostasis in melanoma. Oncol Lett 22:657. https://doi.org/10.3892/ol.2021.12918
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (31571054), Shandong Provincial Key Research and Development Project (2019GSF108224), Natural Science Foundation of Shandong Province (ZR2021MC116).
Funding
This study was funded by the Natural Science Foundation of Shandong Province (ZR2021MC116), National Natural Science Foundation of China (31571054), Shandong Provincial Key Research and Development Project (2019GSF108224).
Author information
Authors and Affiliations
Contributions
JW and JX: designed the study and revised the manuscript. JY: wrote the manuscript. CD and YL: performed experiments and analyzed the data. RL and CJ: assisted with experimental techniques, analyzed the data.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Du, C., Li, Y. et al. Contrasting Iron Metabolism in Undifferentiated Versus Differentiated MO3.13 Oligodendrocytes via IL-1β-Induced Iron Regulatory Protein 1. Neurochem Res 49, 466–476 (2024). https://doi.org/10.1007/s11064-023-04047-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04047-y